An Apple Pie From Scratch, Part IVb: Planets and Moons: Size and Surfaces

|
| DarinK |
It’s all well and good to talk about events at a cosmic scale—galaxies and stars and century-long orbits—but human experience tends to happen within a very local sphere, and that will remain true long after we’ve made our way to other planets, and perhaps even other stars. Worldbuilding isn’t just about building big clockwork mechanisms—it’s also about building experiences.
So in this section I want to explore the question of “what would it be
like to be on this planet?” I’ll tackle it with 3 approaches: First, the
overall physical characteristics of a planet; Second, the many types of
surfaces that a planet might have; and third, the appearance of other
bodies and illumination from them on a planet’s surface.
- Physical Characteristics
- Surfaces
- Solid Surfaces
- Atmospheres
- Hydrogen
- Helium
- Nitrogen
- Oxygen
- Carbon Dioxide
- Carbon Monoxide
- Nitrous Oxide
- Water
- Hydrocarbons
- Halogens
- Sulfur
- Rock
- Others
- Oceans
- Alien Skies
- Calling in the Magratheans
- In Summary
- Notes
Physical Characteristics
Size
Once we have our planets placed in orbits and their masses picked
out, the obvious place to start in defining their characteristics is determining
their size. For a planet of given mass, the radius will depend on the
density, and the density depends on composition.

|
| Mass-radius trends (red line) with typical error (shaded regions) from known planets and exoplanets (symbols). Chen and Kipping 2016 |
Observed exoplanets
can be divided1
into three regimes of mass-radius relationships: Terrestrial planets up to
2.04 Earth masses with small atmospheres and high densities; “neptunes” between 2.04 Earth masses and 0.414 Jupiter masses with densities that
drop rapidly with increasing mass due to larger atmospheres; And “jupiters” above 0.414 Jupiter masses with ever more massive atmospheres but radii
slightly decreasing with mass due to greater compression of the
atmosphere. We can therefore roughly predict the radius of a planet with
any given mass:
m = planet mass (Earth masses)
For planets less than about 0.01 Earth masses, bulk density will be close
to uncompressed density (though you wouldn't expect to find any planets
that small with much hydrogen). For larger planets, we can construct a
mass-composition-radius relation in a number of ways that vary
in complexity and accuracy.
r
= planet radius (Earth radii)
But if you have a little more time to work with and want a bit more
accuracy, we can pick a specific composition and working out a
mass-radius relation from there.
Though there are many types of materials that could conceivably form
planets, we'll simplify matters by dividing them into 4 broad categories,
sorted from most volatile (tending to form low-density gasses
or fluids) to most refractory (tending to form high-density
solids with high melting temperatures):
- Hydrogen, referring specifically to the thick atmospheres of gas giants, though really this is typically a 3:1 mix of hydrogen and helium.
- Water, referring to water present as ice or a fluid on the surface of the planet, not mixed into other materials in the interior. Other volatiles like methane or ammonia may behave similarly, but have not been studied nearly as much.
-
Rock, referring to lithophilic materials that tend to form oxides and appear in the mantle and crust. This is
generally assumed to be mostly a mix of magnesium and silica (SiO2), typically bridgmanite (MgSiO3) or forsterite (Mg2SiO4).
- Metal, referring to siderophilic materials that tend not to form oxides and instead sink to form metal alloys in the core (this doesn't include all metal elements—some like magnesium, sodium, and aluminum are major lithophiles—just those that tend to be present as metal compounds). This is generally assumed to be mostly iron, with some nickel.
Some researchers also define another category, the
chalcophilic materials that
tend to bond with sulfur, but they’re a small portion of most planets,
tend not to form distinct layers, and fall roughly between lithophiles
and siderophiles in their properties.
On the surface, these materials have typical densities of 0.0001, 1,
3.5, and 7.8 g/cm3 respectively, but in the interior of a
planet they can be highly compressed. Earth is about 2/3 rocky mantle
and crust and 1/3 metallic core (even with large oceans and water mixed
into the mantle, water only accounts for about 0.03% of the total mass),
giving it an
uncompressed density of
just under 4.3 g/cm3, but the actual density is 5.5
g/cm3. For a given composition, more massive planets will
have higher densities.

|
| Marc Kushner, NASA GSFC |
Solid Planets
Terrestrial planets like Earth with a clear solid surface are the most
straightforward, as we can ignore the atmosphere and other
volatiles. This approximation2 should be reasonably accurate for rock-metal planets between 0.01 and 100
Earth masses:
R
= radius (Earth radii)
d = distance to horizon (any unit so long as all 3 are the same)
The first rocky material formed would have been
chondritic, named for the
round chondrules, formed from droplets of material that was briefly molten
during accretion, that make up much of its mass. Many asteroids are still
chondritic today. Color varies and albedo of these bodies is typically
0.3-0.6, and density is around 3.5 g/cm3.
Once enough mass has gathered together to form a planet, even a small
one, the heat of formation and internal radioactivity will usually cause
the originally chondritic material to melt and differentiate.
Once the surface cools, it will usually form mafic rocks (so-called because they're relatively rich in magnesium and iron, a.k.a. ferrum), primarily basalt, a grey or black and frankly visually uninteresting rock. Much of the
initial basalt surfaces of our solar system have been ground up by impact
events, but later tectonic activity can cause lava to flood over the
surface, forming flood basalts that are still visible as the dark regions on
the Moon and Mars. Earth has some flood basalts, such as in Iceland, and the
ocean floors are primarily mafic. Albedo is low, around 0.1, and density is
3.0 g/cm3
due to differentiation out of some of the metals.
M
= mass (Earth masses)
fr
= rock mass fraction (where the rest of the mass is metal)

|
| Predicted mass-radius relationships for rock-metal and water-rock planets. Fortney et al. 2007. |
As a guideline, we have
some reason3
to expect that Earthlike planets will generally form with core
masses of about 15 to 40% of their total (rock mass fraction of 0.85
to 0.6 in the above formula), but Mercury has a core mass fraction
of about 70% and we'll discuss the various ways a planet could lose
much of its rocky exterior shortly.
There is some nuance here, of course. These models assume rock with
a similar magnesium/silica mix as on Earth, but in truth
the mix could vary4
and include substantial proportions of other materials like aluminum
and calcium. Even iron may,
in some cases5, be oxidized and mixed into the rocky mantle during formation
rather than forming a distinct core, resulting in a slightly less
dense planet. But in general these issues shouldn't amount to more
than a few percent variance in radius (though if you really want to
explore them and you know a bit about python, it shouldn't be
too difficult to do with
ExoPlex, which can also handle waterworlds).

|
|
Mass-radius curves for planets with distinct iron cores and
"coreless" planets with the iron mixed into the mantle; curves
of the same color have the same bulk composition (aside from
the pure planets).
Elkins-Tanton and Seager 2008. |
Finally, as we'll discuss, so-called carbon planets may have
mantles of carbide rather than oxide minerals, and we can expect
these to have similar densities, but they haven't been studied in as
much detail.
Waterworlds
If there is a thick layer of water that comprises a significant
portion of the planet's mass, things get a bit more complicated.
First off, if the interior is mostly rock and the water at the
surface is ice or liquid below the boiling point, then a simple
mass-radius relationship is
still possible2:
R = radius (Earth radii)
Gas Giants
M = mass (Earth masses)
fw = water mass fraction (where the rest of the mass
is rock)
As a general guideline, bodies formed of icy material
from the outer solar system tend to form with around a
50:50 mix of water and rock, though much of the water can
be later lost.
Incidentally, all these mass-radius relations so far are
included in my
worldbuilding spreadsheet; if you input a planet mass in the “system builder” tab,
it will calculate radius using the simpler 3-regime model
by default, but if you input metal or water mass
fractions, it will assume the rest of the planet is rocky
and calculate radius based on that.
However, if a waterworld has a significant metal core
within its rocky interior, there is no such simple
approximation. We can make a rough approximation by
interpolating between the rock-metal and water-rock
formulas (this is what the spreadsheet does if you enter
both metal and water mass fractions), but if you need a
more precise tool you can refer to the "Manipulate Planet"
tool
here.
But matters become even more complicated if the surface
is warmed to above the boiling point. A large portion of
the oceans may then evaporate into the atmosphere, further
warming the surface through greenhouse heating in a
runaway greenhouse effect. If the planet's surface
gravity and water mass fraction aren't too large, this
water may then all escape into space and leave the planet
dry; otherwise, a long-lived steam atmosphere may form
with no clear ocean surface below it (we'll discuss this
in more detail shortly), something like a gas giant
atmosphere. How large this atmosphere ends up being
depends largely on its temperature, so now we have to deal
with a
mass-composition-temperature-radius relation.
Fortunately,
this paper6
has worked through these factors: taking the mass fraction
of the core out of the combined mass of the rock and core
interior, rounded to the nearest 10%; the mass fraction of
water out of the total mass of the planet, rounded to the
nearest 10%; and the planet's equilibrium temperature
(which I'll discuss shortly) rounded to the nearest 100 K,
you can take the coefficients in the
first file here7
and feed them into this formula:
R = radius (Earth radii)
M = mass (Earth masses)
a, b, c, d = coefficients from the link
e
≈
2.718
The second file also contains a large number of
pre-computed radii, and you can use the error codes to get
a sense of the limits of these approximations for a
particular composition; all should work up to 20 Earth
masses, but are only reliable in the range with error
codes of "0". Lower-mass worlds may be experiencing high
rates of water loss that gradually reduces their water
mass fraction.

|
|
Mass-radius curves for post-runaway waterworlds of
different effective temperatures and water mass
fractions (with pure rock interiors) compared to
those for cool planets.
Aguichine et al. 2021 |
Gas Giants
Much like these post-runaway waterworlds, the size of a
gas giant atmosphere depends largely on the temperature.
But determining a gas giant's temperature is a bit more
tricky, as such huge bodies retain a lot of heat from
formation and continue to produce more as the atmosphere
contracts; Jupiter still produces substantially more heat
than it receives from sunlight. There's a predictable
decline in heating as the planet ages, but high amounts of
solar heating can still have an impact, so we now have to
deal with a rather thorny
mass-composition-insolation-age-radius relation.
Fortunately, the authors of
this paper8
have put together a model accounting for all these factors and released it
as the python package planetsynth. It is a bit large for me to repackage as a standalone program, so
here's a quick rundown on how you might go about using it:
- Install python and use pip to install the numpy and scipy packages.
- Download planetsynth from the above repository (under the green "code" button), unpack the zip file, and run setup.py.
- You can look at the instructions and examples on the repository or copy the script here which will run planetsynth with a short command-line prompt, taking the following parameters:
- Planet mass in Jupiter masses, with a range extending from a body about twice Neptune's mass to a small brown dwarf (the effect of early deuterium fusion isn't taken into account for the latter but this probably only matters for fairly young bodies).
- The bulk metallicity, representing the portion of the planet composed of something other than hydrogen and helium; this is assumed to be around 0.1 for Jupiter and 0.2 for Saturn.
- The metallicity of just the atmosphere, which should be roughly similar to that of the star; so around 0.02 for a sunlike star.
- The stellar flux, which can be calculated as 1367 * [star luminosity relative to sun] / [distance from star in AU]2.
- The age of the planet in billions of years (up to 10 billion years, after which one can assume that the planet's evolution will be very slow).
Other Size Parameters
Now that we have the radius, it’s easy enough to determine surface
gravity and total surface area through the magic of ratios:
g
= surface gravity (Earth gs; 9.81 m/s2)
m
= planet mass (Earth masses)
r
= planet radius (Earth radii)
A
= surface area (Earth areas; 5.10*108 km2)
r
= planet radius (Earth radii)

|
| XKCD |
One other subtle effect is the distance to the horizon; the Apollo
astronauts were reportedly surprised at how close it appeared on the
moon. The exact distance depends on the altitude of the observer, local
topography, and atmospheric refraction, but for the approximation of an
observer on a perfectly spherical, atmosphereless planet:
d = distance to horizon (any unit so long as all 3 are the same)
h
= height of observer
r
= radius of planet
For an observer 2 meters tall, the distance to the horizon is 5.05 km
on Earth and 2.64 km on the moon.
Now, linking radius directly to density assumes that all planets are
spherical. Fortunately this is generally a safe assumption because
even solid materials behave like fluids at the size of planets and so
will tend towards a hydrostatic equilibrium, with the surface deformed such that the gravitational pressure
distributed equally across the whole surface, resulting in a sphere.
The mass required for a planet's gravity to overcome the compressive
strength of rock and force it into hydrostatic equilibrium is about
0.00035 Earth masses, but bodies as little as 0.00005 Earth
masses—corresponding to a radius of about 250 km for rocky material—tend to be spheres as well9 because they were rounded by gravity when they first formed and
partially molten and then were frozen into that shape as they cooled
and solidified. Naturally these thresholds will vary, with less dense
icy bodies tending to become spherical at even lower masses.
But even bodies well above this mass can deviate from spherical due to
their rotation, which causes centrifugal acceleration that
partially counteracts gravity (anyone who tells you centrifugal forces
don't exist is a pedant who doesn't understand the regular use of
mathematically convenient reference frames in physics). This results in an
effective gravitational acceleration that's lower at the
equator (which rotates fastest) than the poles (which remain static), such
that the equator is less compressed and can bulge out further. The
resulting oblate spheroid is shaped such that the
combined gravity and centrifugal force still pulls directly "down" into
the ground at all points on the surface, even though it varies in
strength.
For most planets, this is a negligible effect: Earth is about 0.3% wider
at the equator than between the poles; though Jupiter, which is larger and
spins faster, has a 6.6% difference. But it can become significant for
planets with extremely fast rotation. For such cases, the equatorial and
polar radii
can be estimated10 like so:
ϵ = ellipticity
r = average radius (km)
re
= equatorial radius (km)
rp
= polar radius (km)
d = density factor (where the interior can be approximated as
having a density of [density at surface] * ( [distance from planet
center] / [planet radius] )-d
; d is ~1.09 for Earth and ~1.79 for Jupiter)
π ≈ 3.14159
P = rotational period (hours)
G = 5.166 * 1012 km3
Earth mass-1 hours-2
M = planet mass (Earth masses)
The previous formula for surface gravity will still hold at the
equator, taking into account the increased radius, but
it will now be counteracted by a centrifugal acceleration:
A = centrifugal acceleration (m/s2)
π ≈ 3.14159
re
= equatorial radius (km)
P = rotational period (hours)
(note the units here; to directly compare this to gravity in gs,
multiply gravity by 9.81)
Gravity at the poles will be larger due to the lower radius, but not as much larger as you might expect because part of the planet's mass is now "above" the poles in terms of distance from the planet's center. It can be roughly estimated like so:
Ap
= gravitational acceleration at poles (Earth g)
m = planet mass (Earth masses)
ϵ = ellipticity
r = average radius (Earth radii) (note again I switched back to g here—sorry if that's confusing—and that I'm using the average rather than polar radius because that makes the derivation easier)
If we play around with these numbers, we find that the combination of
Earth's equatorial bulge and centrifugal acceleration only reduces
gravity at the equator by less than 0.6%. If we shortened Earth's
rotation period to 4 hours, the equator would bulge out by over 750 km
relative to the poles and have an effective surface gravity of 0.8 g, while the poles would experience 1.03 g.
At a period of 2 hours, it would bulge out by over 3000 km and
experience only 0.17 g, while gravity at the poles rises to 1.13 g. At a period of about 1 hour and 50 minutes,
centrifugal acceleration at the equator is equal to gravity; any
faster and the planet tears itself apart (though the above method for
estimating the equatorial radius may not hold close to this limit, so
don't take this as an exact prediction).
Intense tidal forces can also stretch a planet from spherical,
causing it to bulge out towards and away from the body inducing the
tidal forces (rather than around the entire equator as for rotation).
The tide height formula I gave in the
last section
should work as a rough approximation of the difference between the
minimum and maximum radii of such a body, and the same approach as
above should work for determining surface gravity at the tip of the
tidal bulge (but the equation for gravity at the poles won't work).
Temperature
The surface temperature of a planet is another major factor determining
both the composition and form of the surface, though it can be very
tricky to work out.
A quick, easy way to get a rough estimate of a planet’s temperature is
to track how total heat gained balances with heat lost: In essence, we
assume that the planet gains heat from the sun based on its
insolation—how much sunlight hits the surface on average,
depending on the star's luminosity and the planet's distance from
it—and albedo—what portion of this light is reflected directly back into space by
the surface. The light not reflected is absorbed and warms the surface.
This will cause the surface to emit thermal radiation into
space, and, per the Stefan-Boltzmann law, increased temperature
causes increased radiation. If the planet radiates more heat than
it gains from sunlight, it should lose heat, cool, and so radiate less
heat; if it radiates less heat than it gains, it should warm and radiate
more. Eventually the surface should reach an average equilibrium temperature where the heat radiated matches the heat absorbed:
Teq = equilibrium temperature (K)
L
= star luminosity (relative to sun)
A
= albedo (0.3 for Earth)
r
= distance from star (AU)
This estimate works decently well for airless, rapidly-rotating bodies,
and replacing the 4 with a 2 will give a decent approximation for the
average dayside temperature of a tidal-locked planet. As an even quicker
approximation, note that, for constant albedo, equilibrium temperature
varies by 1/√(distance from star); so in general we should expect a
planet at 4 AU from the sun to have about half the surface temperature
of a similar planet at 1 AU. But albedo does vary
(generally increasing with distance from the star as more
ices form) so I'll try to give a sense of typical albedo values for each
of the surface types we'll discuss shortly (at least where decent
estimates are available; and note that albedo varies with the angle of
the surface to sunlight, so the total albedo of the planet—the bond albedo—may not be quite the same as the numbers suggest).
But the real trouble comes from atmospheres, which can cause planets to
behave differently from blackbodies. In particular, the
greenhouse effect occurs when the atmosphere contains
certain gasses, like CO2, that are transparent to the
short-wavelength sunlight that warms the planet but opaque to the
infrared thermal radiation out from the planet, They thus reflect much
of that heat back towards the surface and so effectively slow the
overall rate at which the planet loses heat, causing the surface to warm
to a higher equilibrium temperature. A combination of water, CO2, and a few other minor gasses—totaling around 0.3% of the mass of the atmosphere—warms Earth in this way from an equilibrium temperature of 255 K to
its current actual temperature of 288 K.
Some researchers will account for this by incorporating an
emissivity value into the above formula,
representing how much thermal radiation the planet emits compared
to the ideal value estimated by the Stefan-Boltzmann law (for Earth it's
around 0.6), but estimating the emissivity for a particular combination
of atmospheric gasses can be devilishly complicated. Based on
this paper11, I've put together a process for estimating the greenhouse effect and
the specific case of a roughly Earthlike planet with CO2 and water as the dominant greenhouse gasses (though for reasons
we'll discuss later we only need input a CO2 level and then we can
assume this controls water vapor levels) and a resulting average
temperature between 150 and 350 K, which I've added to the
worldbuilding spreadsheet
in the "Hab Planet Temp" tab.
The major downside here is that you need to set a specific albedo,
whereas in reality the albedo should adjust with temperature as
high-albedo ice forms or thaws. I may someday attempt to construct a
more complex model like the one-dimensional energy balance model
described in the paper that accounts for varying ice cover and seasons,
but that will have to wait for another day.
Past that, there aren't really any usable tools for a broader range of
greenhouse gasses. The closest thing I can find to something usable is
HELIOS and that is a
very generous way to describe it. So don't be afraid to
settle for a very vague estimate based on the equilibrium temperature
and a generous fudge factor.
As a final note, planets can receive heat from sources other than
sunlight, most notably geothermal heat from the interior.
I've already mentioned that internal heating can be a significant factor
for gas giants (and planetsynth will determine effective temperature
with this in mind), but with smaller, solid planets it's less of a
concern; Earth's surface receives less than 0.04% of its total heat from
the interior. Jupiter's moon Io, far from the sun and riven by volcanism
caused by its intense tidal heating, still gets only about 1/5 of its
heat from its interior. Even warming small areas with geothermal heat is
a tall order: the Antarctic volcano
Mount Erebus is
one of the few places in the world receiving enough persistent heating
to maintain an open lava lake, and yet is still covered in ice up to the
rim of its crater. Still, as we'll discuss another time, even this small
amount of heat can warm a planet to Earthlike temperatures on its own if
there's a thick-enough atmosphere of greenhouse gasses to trap it
in.
Surfaces
Classifying planetary bodies purely by broad characteristics, while
informative in a pinch, is a bit too simplistic. If we want to know what
a planet will actually be like to visit and live on, then what we really
have to ask is what sort of surface we will encounter—and how many
surfaces, for planets with distinct atmospheres, oceans, and landmasses.
To some extent this is determined by size as well, but there’s a lot of
variation, both observed within our solar system and theorized
elsewhere.
A lot of fictional depictions have a bad habit of depicting
single-biome planets that
are homogenous across their surfaces. It is feasible for a planet’s
surface to be dominated by rock, ice, water, or lava, and to some extent
a desert world is possible, but as more complexity is added
(atmospheres, oceans, tectonic activity) it will tend to lead to more
surface variation. Even Mars, a prototypical desert planet, has rocky
highlands, dusty lowlands, ice caps, and various local features formed
by volcanoes, glaciers, tectonic forces, and water. I would expect any
planet with complex life to have a very diverse surface. Unfortunately,
though, for many types of possible planetary surfaces we can only
broadly speculate.
Let’s take a quick tour of some of the more common types of
surfaces—solid, liquid, and gaseous—that we might expect to
encounter.
Solid Surfaces
Rock
This is the obvious one, but of course there are all sorts of different
kinds of rock. For a system with a composition like ours, rocky material
consists mostly of silica (SiO2) mixed with
magnesium, calcium, and aluminum, though many other
elements appear in varying portions, including some volatiles and
siderophiles like iron.

|
| NWA 3189 Meteorite cross-section in closeup. James St John, Wikimedia |

|
| Basalt. Wikimedia |

|
| Varieties of granite. Jstuby, Wikimedia |
On Earth, tectonic processes cause volatiles to mix with mafic rocks in
the upper mantle at subduction zones, and the resulting magma rises up
through the crust to form
felsic rocks (so-called because they're rich in feldspar,
a sodium/potassium/calcium-aluminum silicate, and silica), primarily granite at first, but
later tectonic activity can form many other igneous and metamorphic
rocks with broadly similar properties. The rocks are even poorer in metals than mafic rocks, lending them a
light grey or white color, or reddish due to alkali metals, and a higher
albedo of around 0.3. They're also less dense, around 2.5 g/cm3, which causes felsic masses to “float” higher on the mantle, forming our
modern continents.
Though felsic rocks on Earth are mostly associated with plate
tectonics, Mars also has regions of felsic crust, and some felsic rocks
may even exist12
on Venus. Typically this requires continuing volcanism in the presence
of water, but other volatiles like chlorine or fluorine may work as
well.

|
| Sandstone. Ester Inbar, Wikimedia |
There is
some evidence4
that rocky exoplanets could have compositions unlike any in the solar
system, particularly varying in the ratio of silica to magnesium.
Silica-rich planets could potentially form fully felsic crusts with more
quartz, which
might be more buoyant14
(especially if rich in sodium as well) and so less prone to subduction and
plate tectonics—though it's hard to be sure given the complexities and uncertainties of
global tectonics (something we will discuss a little more in the next few
posts). On the other hand, more magnesium-rich planets may form crusts of ultramafic rocks like green serpentinite (albedo ~0.3) that are rich in olivine (~0.2).
There are a few other types of rocky planet compositions that are not
considered particularly likely but are worth mentioning just to be thorough;
though the geochemistry here is a bit complex and hasn't really been
rigorously explored so don't put too much trust in my specific
predictions:
- Material at the inner edge of the protoplanetary disk might be heated enough to vaporize away sodium, magnesium, and iron, leaving it relatively enriched in aluminum and calcium. A planet formed from this material16 might lack an iron core and would have a crust rich in reddish (though occasionally yellow or green) garnet, and probably also higher levels of variously colored corundum (which includes red ruby and blue sapphire) and perhaps green epidote and jadeite and blue kyanite. The surface may thus be more colorful overall, with perhaps a reddish tendency, though duller augite, albite, and nepheline may be more common as well. A calcium-rich crust might also form more carbonates, pulling CO2 from the atmosphere, which might make these planets relatively cool if they appear in the habitable zone.
- One possibility I'll bring up a few times in this post is that a white dwarf may be broken apart by a close encounter with a neutron star or other massive object, forming smaller planets. If this occurred with an oxygen/neon/magnesium red dwarf, the resulting planet should form a solid interior of mostly white or green periclase, though the likely presence of carbon may complicate matters and the planet may have a very deep Ne/CO/O2 atmosphere, at least initially.
- Titanium is a common secondary element in rocky crusts, and though there's no particular expectation that it might become more dominant, if it did we might expect more dark ilmenite, red or green titanite, and red, brown, or yellow rutile, famous for its tendency to form long, thin crystals.
Metal
Iron is by far the most abundant metal in the solar system—and
the universe—with nickel a distant second (to be clear, I'm
referring to "metal" in the chemical sense—elements on the left of the periodic table that tend to form metallic
bonds—rather than the astrophysical sense used in the previous posts). Thus,
all rocky bodies in the solar system have iron-nickel cores (though as
mentioned, a planet could
conceivably lack a core5
if all its iron is oxidized during formation, causing it to be mixed in
with the rocky crust). Differentiation during planet formation will typically place metals
under layers of rock and volatiles, so exposed metal surfaces are rare,
but there are a few plausible ways they might develop.

|
| Iron meteorite; the hexagonal crystal patterns form naturally from molten iron in low gravity. Tila Monto, Wikimedia |

|
| Concept of Psyche. Maxar/ASU/P. Rubin/NASA/JPL-Caltech |
But if a planet is large enough to differentiate, a sufficiently
powerful impact could strip away the rocky exterior of a differentiated
body, exposing the metal core; this is
another proposed origin20
for Mercury
as well as the metal-rich
m-type asteroids. The
largest such asteroid,
Psyche, at 0.000006 Earth masses, may be almost pure iron-nickel, at least on
the surface. Attaining such high metal content from impacts becomes more
difficult for larger bodies, as the higher gravity prevents escape of
the ejected material, but it
may still be possible21
to attain bodies of over 90% iron with extremely high-velocity impacts.
The rock need not be completely removed; once the rocky exterior thins
down to 30 km or so, sulfur-rich iron-nickel magma
can erupt22
through the crust and coat the surface.
Alternatively, a planet or moon that passes inside its Roche limit may have23
its outer layers more completely stripped away. A planet in a very close
orbit may also have its rocky exterior gradually vaporized by intense
heating from the star.
However they form, metal-rich planets will be very dense, around 8
g/cm3. Pure metals tend to be grey or silver in color and
have very high albedos, but in most cases the surface will likely be
composed of metal oxides or sulfides with albedos around 0.1-0.2 and
red, black, or other colors.
In addition to exposed cores, a metal-rich layer could conceivably form
on top of rocky crust in
a few different ways15:
- A planet that initially forms with a substantial hydrogen atmosphere may have some rocky materials mixed into that atmosphere as vapor; if the atmosphere is then lost to atmospheric escape, these may then deposit as layers, with sodium possibly depositing last to form a layer of silvery, high-albedo metal.
- An extremely oxygen-rich atmosphere may form large amounts of red or black iron oxide (albedo ~0.2) on the surface, similar to Mars.
- A hot atmosphere rich in water or CO2 could leech metals from the crust and deposit them as minerals like pyrite (FeS2, goldish in color, albedo ~0.1). This may have occurred24 on some highland areas of Venus.
Regolith
This is a broad term describing any loose material over the solid
crust. So it applies to soil and sand on Earth, the ultrafine dust of
Mars, and the surface material of the moon (in principle it could apply
to snow as well, though it's rarely used that way). On Earth and Mars
it’s produced by the weathering of solid rock by wind and water, but on
airless bodies it can be produced by the continuous bombardment of
micrometeorites grinding up the surface; even many asteroids are covered
in regolith.

|
| Left to right: Earth soil (HolgerK, Wikimedia), Lunar regolith (NASA), Martian regolith (NASA/JPL-Caltech/MSSS) |
The moon Titan also appears25 to have regolith formed of tholins (a mix of molecules formed by combinations of nitrogen and hydrocarbons) that form in the atmosphere, deposit on the surface, and then are eroded by methane rains.
Regolith types can be broadly defined by the composition and size of
the average
grain, but our grain size types are calibrated for Earth, where moisture
will tend to bind small grains into larger ones; on drier bodies the
regolith can continue to break down into ever-smaller grains, which
could be a health and mechanical hazard for any future settlers of the
moon or Mars. A typical sand grain in a desert on Earth has around 100
times the diameter of a grain of Martian soil, putting the latter in the
range of “clay”, though it is much less cohesive than Earth’s clay. In
spite of the difference, dunes have been observed on Earth, Mars, and
Titan, indicating that under the influence of similar forces all types
of regolith tend to form similar structures regardless of
composition.
Regolith is produced all across Earth, but most is either washed out to
the oceans by water or held in place by vegetation. Sandy deserts are
caused by an absence of moisture, which leads to an absence of
vegetation, and a lack of static surface material also exposes more
bedrock for weathering. Because this sand is, as mentioned, primarily
silica, the albedo can be as high as 0.4 in dry conditions. Martian iron
oxide dust has an albedo around 0.3, and lunar regolith is close to
basalt, around 0.12. Broadly speaking, all regoliths should be lighter
colored than their source rocks.
Ice
Water ice is the typical surface material for small bodies in
the outer system, but past their respective icelines ammonia
(NH3), methane (CH4), nitrogen (N2), and carbon monoxide
(CO) ice can form as well. In the solar system, water is the dominant component of all icy bodies
that have received close study, but many appear to have some ammonia and
methane as well, Triton and Pluto both have layers of nitrogen covering
parts of their surface, and Mars has large amounts of CO2
ice in its southern ice cap. As we'll discuss, more carbon-rich exoplanets
are conceivable, forming predominantly CH4, CO2, or CO ices. A near-pure CO planet
might conceivably form26
from breakup of a white dwarf.
The iceline for water is estimated to have been around 2.7 AU during
planet formation and is 5 AU currently in our solar system, but for
other compounds it’s harder to pin down: For nitrogen27 and ammonia it’s somewhere inside Saturn’s orbit at 9 AU, for methane
it’s inside Uranus’s orbit at 18 AU, and for CO28 it’s around 30 AU. But the iceline is not a hard limit; many asteroids that formed outside
the primordial iceline but inside the current iceline have surface
layers of dust that protect icy interiors. Inside the primordial
iceline, ice-dominated bodies are unlikely to form, but comets could
deliver at least some ice and this could survive in permanently shaded craters or valleys
near the poles of a planet with low axial tilt. Tidal-locked worlds can
have ice covering their nightsides even in very close orbits, and for cool
atmospheric worlds this may extend into the dayside, forming an “eyeball world”
with ice ringing the warmer center of the dayside.
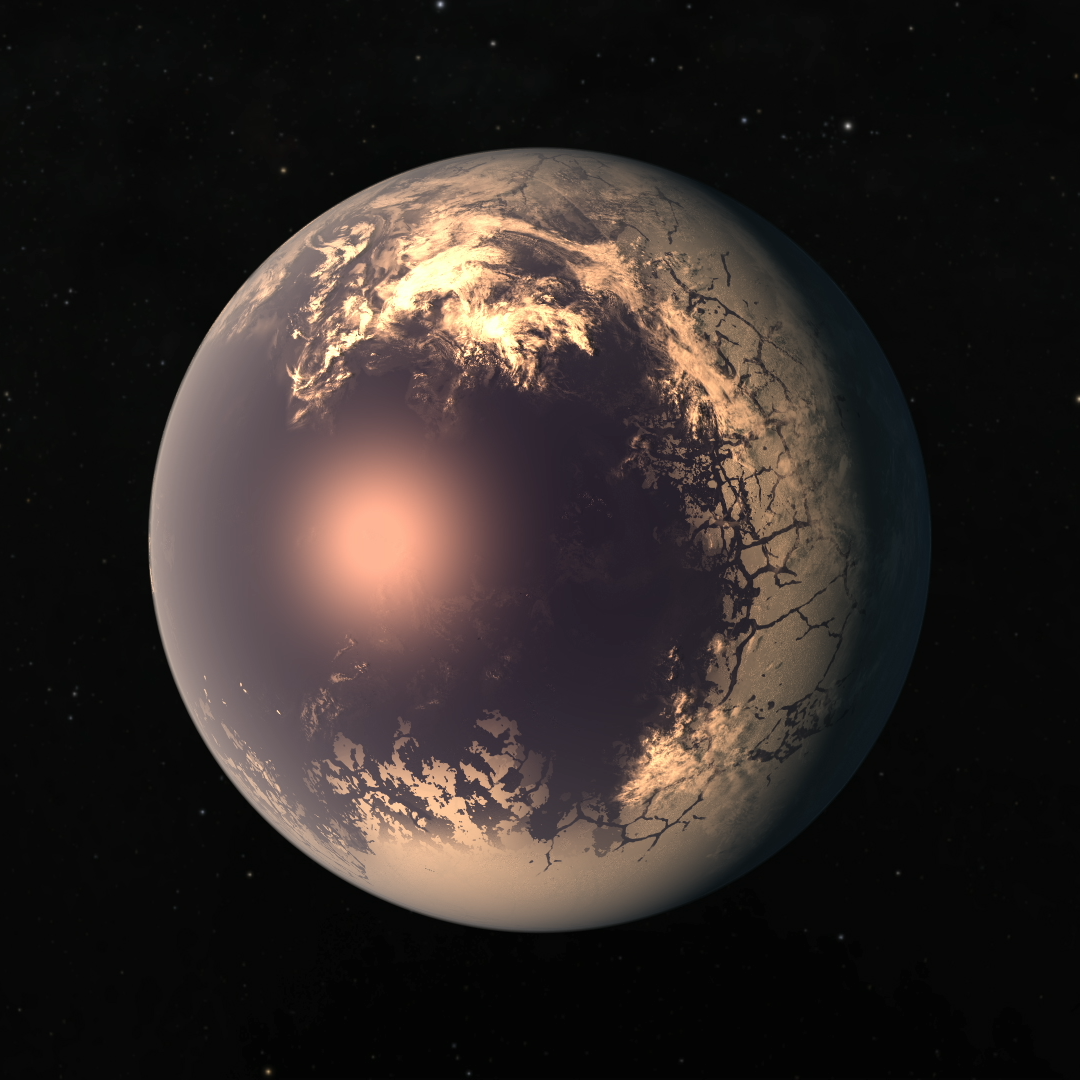
|
| Concept of TRAPPIST-1f. NASA/JPL-Caltech |
An atmosphere will raise the melting temperature of ice, hence the ice
caps of Earth and Mars well inside the iceline. This can work even for
fairly small bodies; were an icy body like Ganymede or Europa to migrate
into the inner solar system, some of the surface ice would sublimate to
form an atmosphere of water vapor. As we'll discuss shortly, much of this
water would escape into space, but these bodies have a lot of water to
lose. As such,
they can survive29
with an icy surface for billions of years in orbits as close as 1.1
AU.
Ice has a lower compressive strength than rock, so it generally can't
form steep mountains like rocks. Once more than about 50 meters of ice
piles up (given Earthlike gravity) it will form a glacier and flow
outwards in all directions unless channeled by rocky features. Glaciers
can be 10s of kilometers thick in their centers, but have very shallow
slopes. If the ice layer is relatively thin (less than 100s of
kilometers) and is underlain by a liquid ocean, the surface will be even
smoother because any topographical features are unsupported. Smaller
bodies with weaker gravity may have more dramatic features, like the
20-km high cliffs of Miranda.
It’s easy to think of an ice-covered world as frozen and dead, but they
can be remarkably dynamic. Many of the icy worlds of our solar system
show evidence of
cryovolcanism, where heat
from the body’s interior causes water to burst through the overlaying
ice just as lava bursts through the crust on Earth. Repeated warming and
cooling of the ice can also cause sections of the surface to rift apart
and slide past each other. On Europa these rifts
may even30
divide into tectonic plates that subduct under each other, just as rocky
plates do on Earth.
|
| NASA/JPL-Caltech |
Triton, Neptune’s largest moon, has a surface layer of transparent
nitrogen ice that causes a sort of “solid greenhouse effect” under
sunlight, heating darker subsurface ice until it melts and bursts
through the surface to form geysers that could
last over a year31
and shoot up plumes of material 8 km high. Triton
also shows evidence32
of cryovolcanism and tectonic rifting, and some flat plains appear to
have been formed by floods of
cryolava of water and
liquid ammonia, analogous to the flood basalts of inner system
bodies. Similarly, large regions of Pluto's surface
have been replaced33
with cryovolcanic activity within the last few hundred thousand
years.
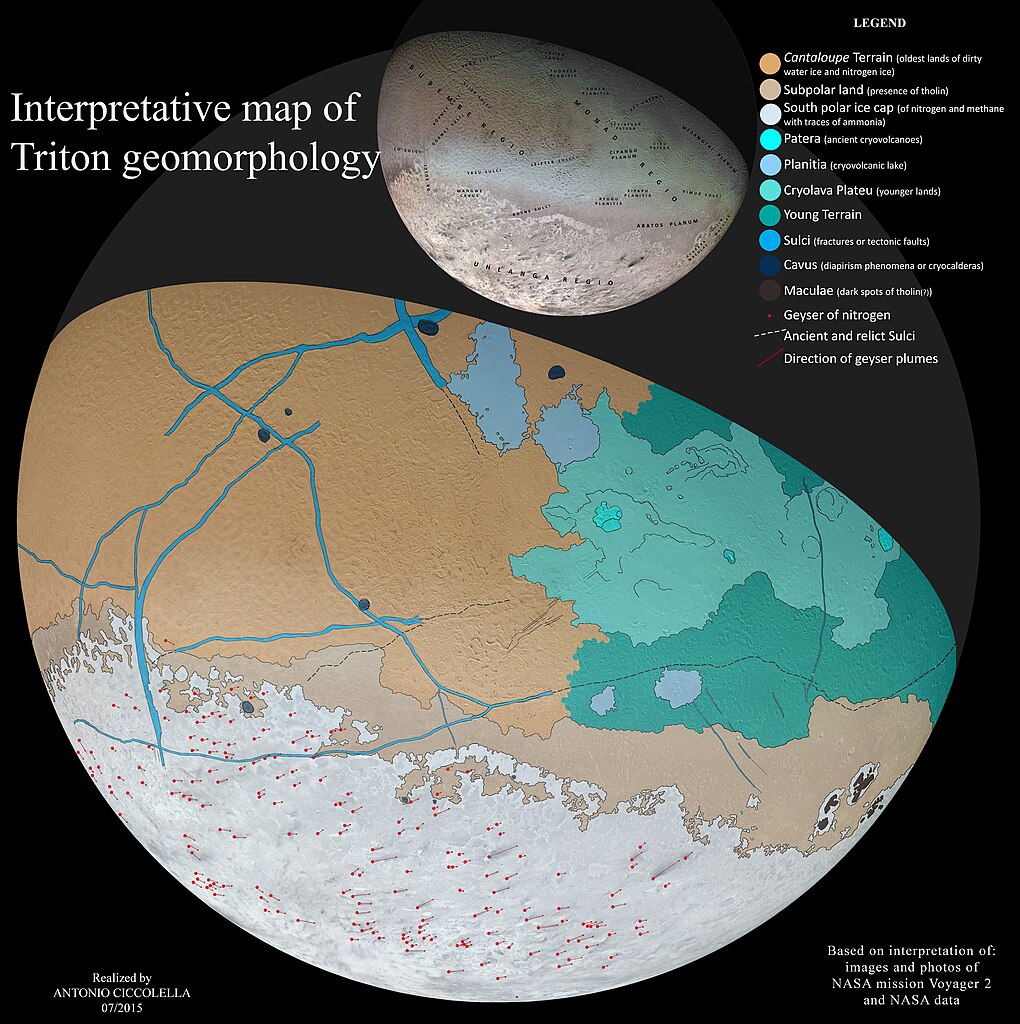
|
| Antonio Cicolella, Wikimedia |
Fresh snow has a very high albedo of 0.8, but partially melted and refrozen ice will typically be closer to 0.6, and “dirty ice” mixed with regolith can be as low as 0.2. These values will all be lower for planets orbiting redder stars. Micrometeorite impacts should tend to gradually coat a planet’s surface with a dusty layer, lowering the albedo, but cryovolcanism and related resurfacing processes can keep the surface covered in fresh ice. Pure water ice is, of course, white or bluish, but methane and nitrogen are more pink, and the ice may be covered by brown or grey dust, red or black tholins formed in a tenuous atmosphere, or byproducts of native life that might be a variety of colors34.
Carbon
Most of what I’ve said so far has assumed a primarily
oxide chemistry: a
silicate and metal oxide mantle, water seas,
and what little carbon there is mostly forming carbonate minerals and
CO2. This is because early in a system’s development, carbon
and oxygen form carbon monoxide (CO), a volatile that is then largely
driven out of the inner system by solar wind. Our solar system has a
carbon/oxygen (C/O) ratio of 0.5—which
should be typical35
of most nearby systems—so formation and then loss of CO from the inner system consumed most
carbon but left plenty of excess oxygen.
However a system with a C/O ratio above 0.8 could retain much more
carbon relative to oxygen, causing the formation of
carbon planets. Individual carbon planets could also conceivably
form in otherwise carbon-poor systems due to
local variations36
in the composition of the protoplanetary disk.
|
|
| Concept of carbon planet. Luyten, Wikimedia |
Such a planet37
would be dominated by
carbide chemistry: a
silica carbide and diamond interior,
graphite surface, hydrocarbon seas, and an atmosphere
likely dominated by some mix of hydrogen(H2), light hydrocarbons like methane(CH4), and carbon monoxide (CO) or carbon dioxide (CO2). Tectonic processes could bring some of the mantle material to the
surface, meaning these planets could have literal mountains of diamond.
However, carbides and diamond
are poorer insulators38
than oxides and likely mix less with heat-producing radioactive materials,
so these planets will cool faster and probably be tectonically inactive
for most of their lives (if not provided with an additional energy source
like tidal heating).
Some planets could also have a mix of oxide and carbide chemistry. If
large amounts of water are added to a carbide planet in the later stages
of formation, it could oxidize the outer layers39, forming a silicate crust like Earth and a diamond and silica upper
mantle; later volcanism could bring some diamond and carbides to the
surface, forming an odd mineral mix, and would likely produce large
amounts of atmospheric hydrogen and methane as a byproduct. Conversely,
carbon may fail to replace oxygen in the mantle during formation and instead40 form a layer of graphite (and, if deep enough, diamond) over a silicate
mantle. Graphite could also precipitate out41 of a hot (>400 K), hydrocarbon-dominated atmosphere, accumulating on
the surface.
It’s hard to judge what the exterior appearance of a carbon a planet
would be, and really it could be as diverse as for oxide planets.
Graphite is very dark grey or black with an albedo of 0.04 (though it
may be higher over the surface of a whole planet with light hitting it
at various angles), but diamond can have a range of colors and much
higher albedo (I can’t seem to find a specific number because most
people have, for understandable reasons, never considered the
possibility of extensive regions of diamond on a planet’s surface, but
I’d expect it to at least be comparable to quartz). But there is a huge
range of minerals that are seen nowhere on Earth but could conceivably
form in a carbon-rich, oxygen-poor environment, ranging from
carbides42
that should behave much as we expect "rocks" to, with high melting
points and stable chemistry, to
various organic materials43
that might more resemble tar, plastics, or other synthetic polymers, and
we know very little about which of these specifically would be
geologically favored.
Sulfur
Sulfur is a bit of an odd element; more volatile than silicate rock and
more refractory than water, but not quite common enough to dominate the
chemistry of the protoplanetary disk like O, C, Si, and Fe do. On Earth
the vast majority of the initial sulfur content sank towards the core
and has remained there, leaving the crust depleted of it, and much the
same is true of most other solid bodies. The exception is in volcanic
regions where sulfur dioxide (SO2) is released into
the atmosphere and concentrated deposits of elemental sulfur can form.
If these minerals aren’t soon buried, they will be weathered by water
and form sulfide or sulfate minerals downstream, in effect diluting the
sulfur in the silicate crust.

|
| Image of Io. NASA/JPL/University of Arizona |
On Io, however, constant widespread volcanism releases huge amounts of
sulfur and there is no water to weather it. As such, Io is covered by
broad expanses44
of yellow and red elemental sulfur and white SO2 ice, with an
average albedo of 0.63. Any world with an Earthlike composition that experienced similar
rates of volcanism for an extended period (which would require high
internal heat, probably only possible from tidal heating by a star or
planet in most cases) could expect similar results. At higher
temperatures, sulfur and
SO2
will evaporate or melt, but there may still be an abundance of sulfide
or sulfate minerals like yellowish pyrite (FeS2), white gypsum (CaSO4·2H2O) and baryte (BaSO4), and orange alunite (KAl3(SO4)2(OH)6).
Glass
Obsidian glass forms when magma cools so rapidly that it cannot
form crystals. This happens occasionally on Earth and some other solar
system bodies, usually forming small fragments, but some researchers
have suggested45
that rapid cooling of magma oceans on exoplanets could form large surfaces
of smooth glass. It would typically be black or grey depending on the
composition and, contrary to what you might expect, have a low albedo of
0.1-0.2.
Vegetation
Obviously I can only speak with confidence about Earth’s vegetation,
but it might be broadly similar for other worlds. Contrary to many
fictional depictions, it’s not realistic for a planet to be entirely
covered in thick forest. Vegetation requires water, and a planet with
enough water to support widespread forests will also likely have large
oceans. Perhaps we could posit floating vegetation spreading onto and
eventually dominating the sea, but that raises questions about nutrient
supply and tolerance of extreme ocean storms. A flat “swamp world” with
global but shallow water coverage isn’t realistic either: For one, there
would be no nutrient-rich mountains or major river systems to supply
these forests; for another, such a world would have to be tectonically
dead, which doesn’t bode well for its long-term habitability. At any
rate, any habitable planet is going to have global weather systems that
lead to wetter and drier regions, and so forest cover should at least
have some regional variation.
But where vegetation occurs, it should be abundant. It’s easy to forget
that the temperate regions of Earth that are now dominated by urban
areas and agriculture had near-total forest coverage before human
activity, and this has been the case most of the time since the
Carboniferous Era. Before then, simpler plants and mats of microbes were
also likely widespread.
On Earth, the albedo of vegetation is around 0.15, lower for thick
forests and higher for grassland. How it might vary and in particular
how plant color might evolve under different stars is a subject we'll
have to explore in depth another time. On Earth, photosynthetic life is
predominantly green but can also be found in red, blue, and purple
varieties. Life exposed to stronger UV radiation
might also evolve46
fluorescent pigments for protection, absorbing UV and emitting it as
visible light.
Strange Matter
Some researchers believe47
that the high pressure inside neutron stars may produce
strange quark matter, composed of condensed quarks rather than
distinct protons, neutrons, and electrons. Collisions between neutron
stars could then tear away pieces of this strange matter, producing
strange dwarfs with masses similar to small stars or even
strange planets. If the strange matter remains stable outside the
pressure of a neutron star, these objects could be astoundingly dense,
over 400 trillion
g/cm3, compared to the 1-10
g/cm3
we expect of planets of normal matter. Alternatively, if the strange
matter is not stable,
it may decompress48
into strangelet crystals (I could not even begin to guess what
these would look like), resulting in a maximum density of a mere 1
trillion
g/cm3
or so and a minimum density of under 10,000
g/cm3.

|
|
Possible mass-radius relations for compact strange matter stars
and relatively "diffuse" strangelet crystal planets.
Alford et al. 2012 |
A strange planet
could also49
form a crust of normal matter. Because this crust will be far less dense
than the strange matter, even at the extreme pressure at the boundary
between them, a large range of densities is possible, down to under 30
g/cm3
(though only at certain masses); so, for example, a strange-matter-core
planet could have 1/4 Earth's mass and 1/3 the radius, for a surface
gravity about twice as strong.

|
|
Possible mass-radius relations for planets with a strange matter
core depending on the density at the base of the normal matter
crust.
Kuerban et al. 2020 |
Similar modelling has not been done for a normal matter crust over a
strangelet crystal core, which may allow for even smaller planets of
moderate gravity. It
may also be possible50
for strangelets and normal atoms to form crystals together, which would
presumably have some intermediate density. A planet with a radius of 100
km, for example, would need a bulk density of 400
g/cm3 to have the same surface gravity as Earth, which may be achievable with
some combination of these materials (though before you get too excited,
the escape velocity of such a planet would be too low to retain much of
an atmosphere).
In any case, such high densities would give these planets very small
Roche limits; a strange planet could potentially orbit a neutron star at
a distance of under 100 km, with an orbital period of under 1
minute.
But it's worth emphasizing again that the existence of strange matter
and its stability outside a neutron star (or that of strangelet crystals) is still speculative, and much uncertainty remains over what exactly
the properties of such materials would be.
Atmospheres
Every planet has at least some thin gas near its surface, but here I’m
concerned mostly with thick atmospheres that can significantly impact
the chemistry of the solid surface and allow for liquid oceans. As a
planet forms, it will typically form a primary atmosphere from
either gasses accreted from the surrounding protoplanetary disk
(moreso the case for gas giants) or gasses released from the initially
molten surface (moreso smaller planets). But as planets age and evolve,
there are a variety of processes that can cause gasses to be gained and
lost; Earth's modern atmosphere bears little resemblance to the one it
likely first formed with.
To start off, volatiles mixed into the crust or deeper interior during
formation can be outgassed into the atmosphere by volcanic activity, which is the primary way by
which atmospheres gain gasses. The gasses produced depends on the
chemistry of both the interior and the crust that magma passes through on
its way to the surface, the latter of which especially may change as the
planet ages and evolves; Earth's volcanos, for example, produced large
amounts of H2 and CH4 when the planet was young but have now shifted more towards CO2 and SO2 (with water more consistently produced throughout). The overall
rate of outgassing will also decline as the planet ages, the interior
cools, and volcanism decreases; but this is only the general trend over billions of years, with plenty of shorter-term
variation.
Once outgassed, whether or not these gasses remain in the atmosphere
depends in large part on the climate; if the surface is cold enough,
some gasses may condense to a solid or liquid form.
Even if this only occurs on a small portion of the surface, the gas
circulating over the surface will continue to collect in this cold trap. As atmospheric pressure drops, this may reduce greenhouse heating or
heat flow across the surface, expanding the cold trap and potentially
leading to total collapse of all or part of the
atmosphere onto the surface. This can sometimes be avoided if the
accumulated solid or liquid can flow out of the cold trap into a warmer
region; as mentioned, even solid ice will flow once more than ~50 m
deep, forming glaciers that tend to reach an equilibrium between melting
at their edges and freezing and snow accumulation in their interiors.
And, of course, a warming climate may instead melt or evaporate
previously trapped volatiles.
Gasses can also react chemically with each other, forming new
compounds and consuming others. We'll have to dig a bit deeper into
the chemistry at some other point, but for now note that atmospheric
gasses (or any materials really) can be classified
as oxidizing, tending to strip away electrons from other
compounds, or reducing, tending to give away electrons, or
anywhere in a spectrum in between; oxygen is a common oxidizer and
hydrogen and carbon are common reducers. Strong oxidizers and reducers
will react with each other until only one remains, so you would not
expect to see an atmosphere containing large amounts of both oxidized
(O2, NO2, SO2) and reduced (H2, CH4, NH3, HCN) gasses at the same time, though small amounts of these
gasses could be appear in a hostile atmosphere if some process is
consistently producing them. Some gasses are more inert (N2, Ar), and compounds of both oxidizers and reducers will tend to
cancel out to produce inert gasses (H2O, CO2, CO), so these can exist in any but the most oxidized or reduced
atmospheres (though their formation may be more favored under one or
the other condition).
Strong oxidizers and reducers will often react spontaneously (though
this depends somewhat on temperature and concentration) but reactions
between more stable materials can be helped along by
catalysts (often metals) on the surface or external
sources of energy; high-energy photons in sunlight or other radiation
sources may cause photolysis, breaking apart gas molecules into
often less stable products that may then recombine in new ways or
react with other gasses. And, of course, life may
metabolize gasses to extract energy or nutrients and then
expel different gasses as waste.
The compounds produced by these reactions may or may not have different
malting and vaporization temperatures and so could condense out of the
atmosphere. Gasses can also react directly with materials on the
surface, often sequestering them in the crust by converting them to solid minerals that may
then be buried. But the materials they react with are consumed as well,
so these crustal "sinks" may eventually become
saturated if all the material has reacted and so no more gas
can be consumed. Volcanic activity can produce new unsaturated crust to
consume more gasses, but at the same time the same volcanism and
tectonic processes (especially subduction that pulls surface
minerals into the hotter interior) can break down minerals in the crust
and release the sequestered volatiles. CO2
in particular exists in Earth's atmosphere in an equilibrium between
rapid sequestration and outgassing.
More permanent is loss of atmospheric gasses to space, from where they
cannot be recovered. In most cases the most important mechanism for this will be thermal escape. Any gas in an atmosphere will have some average velocity based on its
molar mass (a measure of the relative mass of different types of
molecules) and temperature:
vtherm
= typical thermal velocity (m/s)
R
= gas constant; 8,314.4598 g m2 s-2 K-1
mol-1
T
= temperature (K)
mmol
= molar mass (g/mol)
If the average thermal velocity at the exobase—the region at the
top of the atmosphere where gas particles can potentially
reach space without bouncing off other particles—is close to the planet's escape velocity—which,
you may recall, is determined by the planet’s mass and radius—then the planet
experiences
hydrodynamic escape, where
the gas simply flows off the planet like a continuous wind. Escaping
lighter gasses can carry away heavier gasses with them, removing the
entire atmosphere. This appears to be happening to hot jupiters in other
systems, and Earth and Venus
may have51
lost their primary hydrogen-rich atmospheres this way.
But even where the average velocity is below escape velocity, random
collisions will cause some gas particles to greatly exceed the average,
so escape velocity
needs to be52
at least 2-3 times the average velocity at the exobase to prevent
hydrodynamic escape, and even if it's higher all atmospheres still lose
some gas due to
Jean’s escape. The general rule of thumb is that when the average velocity of a gas reaches 1/6 the escape velocity, then the
rate at which it escapes will be great enough that the atmosphere could
become depleted of that gas in less than billions of years. The exact threshold will vary because of complexities in how gasses
distribute in the atmosphere and the potential for outgassing to
compensate for losses, but much beyond this limit and the gas will escape
far faster than any production process can compensate for—save perhaps for brief episodes where a large impact or volcanic event
suddenly releases large amounts of gas, which could then take millions of
years to escape (the same for if an atmosphere is added artificially
during a terraforming process).

|
| Rough chart of gasses that can be retained under given escape velocity and temperature; each colored band represents a different set of gasses that can only be reliably retained by planets in or above that band. Cmglee, Wikimedia |
So for a planet with a composition similar to Earth and a temperature of,
let’s say, 300 K, this leads us to a naïve estimate for the minimum mass
of a planet necessary to retain a Nitrogen-dominated atmosphere of 0.03
Earth masses. However, heating of Earth’s upper atmosphere by solar
radiation can push the exobase temperature up to to 1500 K, and similar
heating should occur for any nitrogen-dominated atmosphere with similar
insolation. This gives us a more conservative estimate of 0.32 Earth
masses for the minimum mass. There’s not a lot of published literature
regarding this question of minimum mass for an earthlike atmosphere, but
I’ve found at least
one more detailed model53
predicting a critical value of 0.07 Earth masses for a nitrogen atmosphere
to last over 4.5 billion years, so my simple model is probably missing
some important factors
regarding the structure of the atmosphere.
It’s a rough, simplified model, but it does generally match what we
observe in our own solar system: massive, cold giant planets in the
outer system with thick hydrogen atmospheres; Earth-mass planets like
Earth and Venus or smaller but colder bodies like Titan with little
hydrogen but thick atmospheres of heavier gasses; sub-Earths and dwarf
planets like Mars and Pluto with thin atmospheres; and small dwarf
planets and minor bodies with no more than trace atmospheres.
Atmospheres can also be lost due to
non-thermal escape. Massive
impacts can toss superheated plumes of gas into space (and may also heat
the surface, quickening thermal escape). Frequent impacts during
formation or the Late Heavy Bombardment may have helped remove the
primary atmospheres of all the terrestrial planets and a few late
impacts may in particular have contributed to Mars's currently quite
thin atmosphere. On the other hand, comets may also deliver large
amounts of volatiles to dry planets, potentially more than they cast
away, and can
substantially alter54
the surface chemistry while they're at it.
Planets without a strong magnetic field can also lose gasses due
to the impacts of solar wind particles, a process called
sputtering, but the
importance of this escape mechanism is often vastly overstated; a
magnetic field prevents sputtering (caused by charged solar wind
particles), but does essentially nothing to stop thermal escape (caused
by light, which is unaffected by magnetic fields, so it doesn't stop UV
or gamma radiation either), and there are few situations where the
former could causes significant losses and the latter wouldn't (perhaps
a planet orbiting a pulsar or a moon in the radiation belts of a gas
giant). Besides which, even if a planet lacks an
intrinsic magnetic field produced by the interior, an atmosphere
can create its own induced magnetic field through interaction
with solar wind, as is the case for Venus. This may even be preferable;
because intrinsic magnetic fields direct some solar wind particles
towards the poles, a weak intrinsic field
may actually increase55
atmospheric losses.
An important point to bear in mind is that most of the processes we've
discussed are quite rapid compared to the billions of years a planet may
remain geologically active or habitable, so rather than gradually adding
or removing gasses they tend to quickly move towards equilibrium, where
gains from atmospheric sources are matched by losses to atmospheric sinks
(as a gas builds up to higher partial pressure this will usually quicken
sequestration and escape, so this doesn't require any wild coincidences).
On early Earth, for example, oxygen sequestration far outpaced production,
so oxygen was virtually absent from the atmosphere for billions of years;
but production rose and sequestration declined until the former surpassed
the latter about 2.4 billion years ago, at which point oxygen rapidly
became a major component of the atmosphere. Levels continued to fluctuate
thereafter, but mostly due to shifts in gain and loss rates, shifting the
equilibrium.

|
| Rough diagram of the likely most common atmospheric gasses based on planet mass (which controls escape velocity) and solar irradiation (which controls surface temperature) due to various escape mechanisms; the exact boundaries here are inexact because they can vary depending on numerous minor factors. Lichtenberg et al. 2022. |
The balance of all these potential sources and sinks and the typical
chemistry of rocky planets gives us some sense of which types of
atmospheres we should expect to be most common. But there's still plenty
of room for variation, and a fair bit of uncertainty due to how complex
the interactions of different mechanisms can become. Water, for example,
appears to have a high-enough molar mass and inert-enough chemistry to
be retained in the atmosphere any Earthlike planet, but photolysis in
the upper atmosphere can break it into hydrogen, which easily escapes,
and oxygen, which will likely sequester on the surface. However, a
nitrogen-rich atmosphere prevents this by forming a cold trap, a
layer of the atmosphere cold enough to cause water to condense and rain
back down to the surface, removing it from the atmosphere for the moment
but preventing permanent escape to space. Recognizing these processes
(not to mention something more complex like the carbonate-silicate
cycle) requires having a fairly detailed model of Earth's surface
conditions, which we just don't have for many of the more speculative
cases we'll be discussing today.
This is especially relevant to the common assumption that a bigger
planet with higher gravity must always have a thicker atmosphere. There
is some truth to this; in the extreme cases, a sufficiently small planet
will lack the gravity to retain any more than a trace atmosphere and a
sufficiently massive planet will accrete enough hydrogen as it forms to
become a gas giant. Even closer to Earth's mass, larger planets
should generally have hotter interiors and more
sustained outgassing from volcanic activity (though that activity could
also cause more sequestration of certain gasses) and atmospheric escape
of all kinds slows for greater escape velocity, so we should expect some
correlation (higher gravity also holds the atmosphere closer to the
surface, creating a higher surface pressure for a given atmospheric mass
per surface area). But different planet chemistries and histories can
create vastly different sources and sinks that can create much greater
variance in the ultimate atmospheric mass and composition than the
influence of a small difference in mass. Within our own solar system,
Venus and Titan both have greater surface pressures than the more
massive Earth. Even if we restricted ourselves to more Earthlike
temperatures and atmospheres, I wouldn't be especially surprised to
encounter a world half Earth's mass with a greater surface pressure or
one twice Earth's mass with less.
Presuming you do manage to settle on a particular atmospheric
composition, a few final notes: First, for a planet with a given surface
pressure, pressure will fall more quickly with altitude for planets with
higher surface gravity and average molar mass. More precisely:
p
= pressure (bar)
p0
= pressure at 0 altitude (bar)
e
≈ 2.71
g
= surface gravity (Earth gs)
h
= altitude (meters)
m
= gas average molar mass (g/mol)
For convenience I'll use the unit of bar for pressure
throughout this post, which is about the average sea level pressure on
Earth but slightly less because it comes out to more convenient SI units
(100 kPa rather than 101.3 kPa) and so is commonly used by atmospheric
scientists (but the above formula should work with any pressure
unit).
There is no sharp boundary for the top of an atmosphere; the density of
gasses continuously drops until it reaches that of the interplanetary
medium. Rough boundaries can be marked where the chemistry and behavior
of that thin medium is dominated by the planet rather than the star, but
this boundary lies far beyond the point where life could exist or
orbiting objects would stop experiencing significant drag. By convention
the boundary of space is often set at the “Karman line” at 100 km
altitude above Earth, where the pressure is roughly 0.000001 bar, though
even at 2-3 times this altitude there is still enough thin gas to cause
satellite orbits to decays within a few days or weeks. The exobase where
Jeans escape occurs is at around 500 to 1,000 km.
Atmospheres can warm a planet due to the greenhouse effect, but thick
atmospheres can reflect away most sunlight before it reaches the
surface. Very broadly, an atmosphere with Earth’s surface pressure
should lead to higher surface temperatures than one with a pressure 100
times higher or lower, but there are plenty of exceptions, especially
given that very thick atmospheres are often composed mostly of
greenhouse gasses.
Hydrogen
By far the most common gas in the universe, and we expect that pretty
much all large planets should initially form with a
hydrogen/helium-dominated atmosphere. But it's also the lightest gas by
molar mass and so escapes pretty easily. Generally speaking, we expect
most bodies above 2 Earth masses should have thick hydrogen atmospheres
and most smaller ones should lack it, but there's plenty of grey area:
even gas giants could lose most of their atmospheres through impacts or
intense solar heating, leaving their bare cores as
cthonian planets of 10 Earth masses or more, and planets below
Earth's mass could keep their hydrogen atmospheres to form
gas dwarfs if sufficiently cold (below ~100 K).
Within a gas giant, most of the hydrogen does not exist as a gas. Using
Jupiter as a model, the visible surface is 50-kilometer-thick region of
clouds, blocking most light from reaching the interior. Below that the
pressure and temperature gradually increases, reaching levels so high
that there’s no sharp distinction between gas and liquid; the hydrogen
exists as a
supercritical fluid, with a
mix of gas-like and liquid-like properties and becoming more liquid-like
with increasing depth and pressure. Around 15,000 kilometers down—1/5 of
the radius—the hydrogen is expected to behave as a metal, though still
not a solid. It's still unclear whether there is a solid core of rock,
ice, and metal under all this, or if this material has all dissolved
into the metallic hydrogen.

|
| Kelvinsong; Wikimedia |
Ice giants have outer layers of hydrogen gas and supercritical fluid,
but unlike gas giants their interiors probably lack metallic hydrogen
and are instead dominated by supercritical phases of the other volatiles
that make up the bulk of their mass.
Terrestrial planets like Earth and Venus likely form with hydrogen
atmospheres of 10s to 100s of bar. On Earth, this was probably completely
lost within a few 100 million years, but planets just a bit larger or
colder could plausibly retain some hydrogen; exactly how much will depend
on a variety of processes in planet formation and later evolution, so
essentially anything between a thin remnant atmosphere and one approaching
that of a gas giant is conceivable. Even if the primary hydrogen-dominated
atmosphere is lost, a more reducing internal chemistry may cause volcanoes
to outgas hydrogen, maintaining a hydrogen-rich atmosphere
even for smaller planets56. In any case, hydrogen can act as a greenhouse gas in the presence of nitrogen or other
gasses, and this may have helped57 warm the early Earth. But it is also a strongly reducing, meaning it
cannot exist alongside oxygen, which may pose issues for the development
of complex life.
If thick enough to obscure the solid surface, then the
outward appearance58
of an H2/He atmosphere will depend on the clouds and hazes
that form at the top of the atmosphere, which in turn depends mostly on
temperature but also on composition and surface gravity. There are many
possible variations, but here's a quick overview of the general trends59
that we should expect:
_flatten_crop.jpg/600px-Neptune_-_Voyager_2_(29347980845)_flatten_crop.jpg)
|
| Image of Neptune. NASA / JPL / Voyager-ISS / Justin Cowart |
The coldest atmospheres, below 100 K, will have low-lying methane
clouds, contributing to a blue color, though this is also largely due to
Reyleigh scattering (the same effect that makes Earth's sky blue).
Albedo is around 0.3.

|
| Image of Jupiter. NASA, ESA, A. Simon (Goddard SpaceFlight Center) and M.H. Wong (University of California, Berkeley) |
At around 100 to 150 K, ammonia clouds appear with traces of carbon and
sulfur compounds that result in the yellow-brown color. Lower-mass
giants like Saturn may have a high haze that gives them a homogeneous
appearance, but higher gravity can reduce this haze and show cloud bands
colored red, orange, and gold due to production of unstable trace gasses
by sunlight, as for Jupiter. Albedo is around 0.4.

|
| Concept of HD 189733b. NASA, ESA, M. Kornmesser |
At higher temperatures
of around 150 to 350 K, water clouds like Earth may appear that are
predominantly white with an albedo as high as 0.8. At higher
temperatures, these clouds may disappear and the color may return
again to blue due to Reyleigh scattering. However,
recent research60
suggests that at 250 to 700 K, a sulfur haze may form above any
clouds, turning the planet orange with an albedo of 0.6.

|
| Illustration of HD 149026b. NASA/JPL-Caltech/T. Pyle (SSC) |

|
| Appearance of the day side of hot giants depending on temperature and cloud composition. A real planet would have a mix of these clouds, with those in the lower rows tending to form highest and so be more prominent at lower temperatures. NASA/JPL-Caltech/University of Arizona |
(You can also check out this
more detailed breakdown62
made by
the folks over at
Orion's Arm, which contains some more speculative options.)
Though atmospheric nitrogen doesn’t interact directly with most life, it
has 2 important roles in maintaining Earth’s habitability: First, it
creates a cold trap in the
atmosphere that causes rising water vapor to condense and rain back down,
keeping it from the upper atmosphere where it might escape; Second,
nitrogen gas can be “fixed”
by lightning or microbes into vital nutrients like ammonium (NH4+) and nitrate (NO3-). It’s hard to say
if this makes atmospheric nitrogen necessary for life, but at any rate it
certainly appears helpful. In turn, oceanic life
appears to encourage71
higher atmospheric nitrogen.
Nitrous Oxide
The range of temperatures and pressures required for stable liquids varies greatly, but for our purposes we can generally summarize the extremes with two key points: the triple point, which represents the minimum temperature and pressure at which the liquid can exist (usually; a few materials like water can remain liquid at temperatures below their triple point, but only slightly except at very high pressures); and the critical point, which represents the highest temperature at which liquid can exist and the minimum pressure to remain liquid at that temperature.
Hydrogen Cyanide
None of these scenarios seem especially likely or have been explored in much depth, but they are tantalizing as HF and HCl are similar to water in many ways but abundant halogens could allow for intriguing new forms of biochemistry, which we'll discuss in a later post.
Above 3500 K, the atmosphere is thick and hot enough to
melt the whole surface139
of even a tidal-locked planet. Albedo of a molten rock surface may be around 0.1, though alkali and
silicate clouds may darken the planet further—not that they'll look dark
under such intense light, and at such high temperatures the planet
itself will glow red.
Helium
All hydrogen-dominated atmospheres will contain some helium as well,
though in gas giant atmospheres the helium may
tend to rain out63
into the metallic hydrogen layer, leaving the upper atmosphere
relatively poor in helium compared to the typical 3:1 mix.
Under
the right conditions64, a Neptune-like gas giant in a close orbit of its star may lose most
of its initial hydrogen atmosphere but retain its helium. The resulting
planet will likely have a whitish color and high albedo.

|
| Concept of GJ 436b. NASA/JPL-Caltech |
It's also conceivable that a planet of just the right mass to retain
helium but not hydrogen might lose its primary hydrogen-helium
atmosphere initially and then gain some helium later as a byproduct of
radioisotope decay,
though this will probably be only a minor component of the resulting
atmosphere unless it is very thin.
Alternatively, a white dwarf star
may lose most of its mass65
in a close encounter with a neutron star or be broken apart in some other
way, leaving a small remnant with a helium- or carbon-dominated
atmosphere.
As a quick aside, a "hot Neptune" too small or hot to retain helium
will likely end up66
with an atmosphere of either water, hydrocarbons, or one of the two mixed
with CO or CO2, depending on the C/O ratio and temperature. In principle, a very
carbon-poor planet with complete hydrogen loss could be left with mostly
oxygen, though that may depend on more details of the planet's chemistry
than was modeled in that source.
Nitrogen
The main component of Earth’s atmosphere (78% by volume) as well as
that of Titan (95%) and a major component of Venus’s atmosphere (3.5%).
A nitrogen-rich atmosphere
has also been proposed67
for early Mars, though it's one of several competing models. That
nitrogen is so common in the solar system across rather different
atmospheres likely indicates it's common elsewhere, likely initially
delivered to forming planets by ammonia (NH3)-rich comets and
then outgassing later. Plate tectonics or other processes that oxidize
the upper mantle
may encourage68
this outgassing, and indeed Earth's atmospheric nitrogen
appears to have increased69
over time as its geology and chemistry evolved. But given that Venus has
3 times as much atmospheric nitrogen as Earth, the ultimate level may
vary greatly even between similar planets.
N2 is relatively inert compared to most other atmospheric
gasses—the two nitrogen atoms are joined by a strong triple bond,
preventing most chemical reactions—and while
it does appear70
to cycle into the interior over long periods, it isn’t often incorporated into surface minerals like oxygen,
CO2, and water often are. This also means it can exist in
stable mixes with many other gasses.

|
| Image of Earth. NASA |
Though Earth is blue primarily due to its oceans, nitrogen contributes
as well. Any “colorless” gas will tend to appear blue in thick
atmospheres, due to Rayleigh scattering. Even icy or desert worlds
may appear blue72
if they have thick nitrogen atmospheres.
Oxygen
A major component of Earth’s atmosphere (21%) but absent from other
atmospheres in the solar system save for the very thin, transient
atmospheres of some icy moons (due to photolysis of the surface ice).
Free oxygen’s (O2) rarity is due to its highly reactive
nature, and on Earth it’s only sustained by the action of photosynthetic
life. But though oxygenic photosynthesis evolved around 3 billion years
ago, consistently high oxygen levels
have only existed73
for the last 6-800 million years. Before then, excess oxygen was mostly sequestered by reactions with
surface materials to create oxide rocks and, save for a brief period
around 2.3 to 2 billion years ago, concentration was below 1/100 of
current values. How oxygenic life eventually came to overcome these
oxygen sinks is a complex question, possibly relating to gradual
geochemical changes in the surface and
the growth of the continents74, but it's a subject we'll have to leave for another time.
However, there are a few conceivable pathways by which large amounts of
free oxygen could be produced without life, predominantly by
photolysis; breakdown of compounds by light.
First off, I mentioned that "hot Neptunes" might form oxygen-rich
atmospheres if they lose enough hydrogen and I'll mention later that a
planet heated to the point of melting its surface might release some
oxygen, but I'm not confident that these predictions would stand up to a
deeper analysis of these planets' chemistry nor that these atmospheres
could survive if the planets cooled to an Earthlike temperature.
UV radiation can photolyze water (H2O) into H2 and O2. The H2 escapes to space much more easily, leaving excess O2 behind. The cold trap formed by nitrogen (or a similar gas like argon) limits the
rate at which water reaches the upper atmosphere, where it can be exposed
to UV and photolyzed. A warm-enough atmosphere could allow significant levels of water to pass
the cold trap; this is called a moist greenhouse, as the large amounts of atmospheric water will cause a strong
greenhouse effect, warming the surface to over 340 K and causing rates of
water loss great enough to dry out the surface in a geologically short
period of time. This may occur for a brief period as planets cool after
formation, but the molten early crust will typically absorb all of the
produced oxygen. But if a planet is fairly dry75
(with less than about a third the water as Earth), the crust may solidify
faster, leaving significant oxygen that could persist for billions of
years if the planet is relatively inactive and doesn't produce much more
crust that would sequester it.
At the opposite end of the scale, a waterworld with global oceans over 50
km deep may suppress the formation of new crust, similarly leaving no
oxygen sinks, such that even the low rates of photolytic oxygen production
with a cold trap would eventually accumulate to high levels over billions
of years.
Alternatively, an otherwise Earthlike world without any nitrogen
would form a water-dominated atmosphere without a cold trap. Oxygen
would form by photolysis until it formed a cold trap, which should occur76 at roughly 0.15 bar of oxygen—slightly less if there is some small
amount of nitrogen or argon. Some water would be continuously lost, but over 4 billion years
this would only amount to about 1/4 of Earth’s current oceans.
But the best prospects for abiotic oxygen may be for planets of red dwarf
stars. First off, planets of mid-range red dwarfs77 could achieve a moist greenhouse at much lower temperatures, as low as
280 K, and retain oceans for billions of years, allowing for a planet with
both habitable temperatures and significant abiotic O2. Even without a moist greenhouse, the high UV output of a young red
dwarf star may photolyze large amounts of water in spite of the cold
trap,
potentially producing78
100s of bar of O2, though how much of this would be sequestered hasn't been
investigated.
CO2 can also be split to produce oxygen79
around red dwarfs, with or without water present, potentially reaching
over 1 bar of O2, depending on
the CO2 level (~0.1 bar of CO2 is required for 1 bar of O2 and 5 bar of CO2 could give 100 bar of O2) However, a smaller amount (~0.05-1 bar) of carbon monoxide (CO) is also
produced, which may be problematic for human habitation (any native life
would presumably adapt to tolerate it).

|
| Several scenarios for production of O2-rich atmospheres. Meadows et al. 2017 |
Finally, large amounts of titania (TiO2)
delivered to a planetary surface by meteorites
could catalyze80
the photolysis of water by lower-energy near-UV light that can more
easily penetrate through the atmosphere to reach the surface.
Once oxygen does reach high levels, it will produce an
ozone (O3) layer
in the upper atmosphere that can reflect away harmful UV radiation, and
it will allow
aerobic
(O2-consuming) life to develop into more complex and
energy-intensive forms. If complex life does evolve and vegetation
becomes a major feature of that surface, the flammability of that
vegetation
may control81
O2 levels thereafter; on Earth, vegetation is difficult to
burn below 16% atmospheric oxygen, allowing levels to rise without being
consumed in large fires, but far more flammable above 22%, consuming
excess oxygen and bringing levels back down
(though the greater diversity of vegetation in nature than was used in
this study and likely evolution of fire resistance in response to oxygen
levels may widen these bounds). Perhaps for this reason, oxygen levels
have remained fairly close to 20% (~15-30%) since the appearance of
widespread forests 350 million years ago.
Like nitrogen, oxygen should appear blue in thick atmospheres.
Carbon Dioxide
The primary component of Venus’s (96.5%) and Mars’s (95%) atmospheres.
On Earth it’s a minor gas (0.04% and rising) but it is the primary gas
responsible for the surface temperature, due to the greenhouse effect.
Technically water is Earth's primary greenhouse gas, but because water
vapor condenses to a liquid so easily, it would be unstable on its own
and is in effect controlled by CO2 levels, amplifying
its effect.
CO2 should be pretty common as an atmospheric gas, as it should be
regularly produced in the interiors of planets that are not heavily
reduced and then outgassed by volcanoes. If there is water in the
atmosphere, the gasses will mix to produce carbonic acid (H2CO3) that will react with surface materials (primarily calcium on Earth) to
produce carbonate minerals. Feedbacks in this process control the surface
level of CO2, but given their profound role in habitability, we’ll leave a detailed
discussion of them to the next post.
But if there is no water, suitable surface materials to react with, or
other process to sequester CO2, it can accumulate in the atmosphere to high levels, resulting in
something like Venus's 90 bar of CO2, with a strong greenhouse effect producing high surface temperatures
(~700 K on Venus). This is expected to be the typical result of a
moist greenhouse or runaway greenhouse scenario,
where the surface becomes hot enough to force water into the upper
atmosphere, where it is then lost to space. This can occur for a planet
inside the inner edge of the habitable zone (which we'll discuss in the
next post) or even within the inner habitable zone if CO2 production outruns sequestration and levels rise high enough. But
with the weaker solar heating past around 1.36 AU (from a sunlike star),
CO2 alone
can never get the surface hot enough82, as the levels required for that much greenhouse heating are higher than
the levels at which CO2 clouds form, increasing the planet's albedo and cooling it (likely
to the point of freezing over).
When a hot, CO2-dominated atmosphere does form, what little water that remains may react
with volcanic sulfur compounds to produce thick, beige-white clouds
of sulfuric acid (H2SO4), as we see on Venus.
Various other hazes83
of sulfur-based or organic compounds could form for similar planets,
likely similarly beige, orange, or brown, and even white water clouds
may be possible84
for planets with less active volcanism or cooler stars.

|
| Venus in true color; most images you see with clear cloud formations are false-color composites including UV light. NASA |
Though it warms the surface, CO2 also cools the upper
atmosphere; Venus actually has a colder upper atmosphere than Earth.
Paradoxical though this may seem, the same properties are responsible
for both effects: near the surface, CO2 absorbs heat from the
ground and radiates it back down, trapping heat in the lower atmosphere;
in the upper atmosphere, CO2 absorbs heat from the
surrounding gasses and efficiently radiates it into space. Because the
upper atmosphere is so thin, this cooling has almost no direct impact on
surface temperature, but it can slow down thermal escape of atmospheric
gasses. In combination with CO2’s high molecular mass this
means that, at a given surface temperature, the minimum mass limit for a
body to retain a CO2-dominated atmosphere should be lower
than for an nitrogen-dominated atmosphere.
Carbon Monoxide
The chemically reduced counterpart to CO2. CO is fairly common cosmically, forming a significant portion of the
ice in many comets, but is vanishingly rare on Earth and most other large
bodies because of sunlight-aided reactions with water vapor that oxidize
it to CO2. Still, there are a couple scenarios where it might be more
stable:
As previously mentioned, for planets of red dwarfs, photolysis in
atmospheres with at least 0.1 bar of CO2 could provide79
a steady source of CO, enough to maintain over 0.1 bar even with water
present (because the weaker UV light from red dwarfs makes CO and water
less prone to react), and larger amounts might form on a dry world.
A larger amount of oxygen should usually form as well, but that
could conceivably85
be sequestered on the surface.
Similarly, if life produced large amounts of organic
carbonyls (a family of organic molecules including
formaldehyde (CH2O)), their photolysis could produce CO as a byproduct,
which could accumulate86
to high levels for planets of red dwarfs, especially in H2-rich atmospheres (the carbonyls themselves dissolve easily in water or
hydrocarbons and so are unlikely to build up to high levels in the
atmosphere).
Alternatively, strongly reducing interior chemistry, like that of a
carbon world, may cause volcanoes to outgas CO in place of CO2, alongside CH4 or H2 as we'll discuss shortly. On a carbon world, there may also be no
water to react with the CO. This could also occur for a less fully reduced
world with a dry surface and volcanism from its deep interior; our moon
may have hosted87
a thin (~0.01 bar) atmosphere of CO and sulfur during peaks in its
volcanic activity.
Finally, as mentioned before, a planet formed by breakup of a white dwarf
might be composed of mostly CO, with a CO atmosphere over layers of CO
liquid, supercritical fluid, or ice.
Compared to CO2, CO is not a greenhouse gas and condenses at a much lower temperature,
so there may also be a range of conditions where CO2
and water freeze out of the atmosphere but CO remains.
CO is fairly toxic to complex life on Earth, but there's no strong reason to expect life couldn't adapt elsewhere; some microbes86 consume or produce CO in much the same manner as most surface life does with CO2.
CO is fairly toxic to complex life on Earth, but there's no strong reason to expect life couldn't adapt elsewhere; some microbes86 consume or produce CO in much the same manner as most surface life does with CO2.
Nitrous Oxide
Nitrous oxide (N2O) is cosmically rare and rather reactive, but is produced by life
during nitrogen use. It's usually promptly converted back to N2
by metal catalysts or photolysis by UV, but higher biotic N2O production and a lower UV (so a K- or M-type star) could allow it88
to build up to over 0.001 bar, perhaps higher for a planet further out
in the habitable zone. So it will probably never be a dominant atmospheric gas, but I thought
it worth highlighting because N2O is a strong greenhouse gas and degrades ozone, so various interesting
feedbacks could result, either helping life to maintain ideal
temperatures for itself or causing some cascading disaster where a
sudden increase or decrease in N2O production changes the climate.
Similar levels of N2O could also potentially be caused by photochemistry in a more reducing
atmosphere (in particular containing CH4) orbiting a more active star with more flaring activity, as
may potentially have happened89 for the early Earth.
Water
Some water will exist in the atmosphere of any planet with surface
oceans of water like Earth (~0.25% average, locally 0.001-5%). The
amount of water in the atmosphere is largely controlled by temperature,
and, as water is a greenhouse gas, this leads to an unstable feedback:
at low temperatures, water mostly rains down to the surface and other
greenhouse gasses like CO2 are required to keep temperature high enough to prevent the planet
freezing over completely; if temperatures get high enough for water to be
a major component of the atmosphere, the strong greenhouse effect warms
the surface further, evaporating more water, and so on in a runaway that
warms the surface well past what we might consider habitable. Earthlike worlds may regularly pass through a stage of steam atmospheres
in this runaway state, outgassed from the molten interior during
formation, with the water quickly condensing and raining down as the
surface cools; but if interior heat remains high and this stage lasts more
than a few million years, it could cause significant loss90 of the water to space.
However, if a mature, cool planet has no other gasses like N2 in the atmosphere (or very little of them) and the planet doesn't
get too much solar heating, then the low pressure will encourage enough evaporation for a thin but
stable water-dominated atmosphere (~0.01 bar91
for an Earthlike surface temperature, equal to the
saturation pressure). Much of this water may escape to space, but as mentioned, enough oxygen
may eventually accumulate to form a cold trap, or a waterworld with deep oceans may just have a lot of water to lose, surviving for billions of years92
without losing a significant portion of its total mass. As mentioned
earlier, a small icy body may also form a water-dominated atmosphere if it
migrates inwards, though it would remain very thin, less than 0.005 bar,
until the ice surface melts and a runaway begins.

|
| Concept of Kepler-69c. NASA/Ames/JPL-Caltech |
Really, though, most planets that form with large amounts of
water should also incorporate some ammonia (NH3), which should then photolyze to N2 and H2, the latter of which may or may not escape depending on the
escape velocity and temperature. So
we should expect93
most temperate waterworlds to have atmospheres dominated
by N2 or H2, or perhaps occasionally CO2, CH4, or O2.
But what about a planet that does go through a runaway? As
mentioned, a world like Earth would lose its oceans fairly quickly
and form a dry, CO2-dominated atmosphere, but a waterworld can sustain a hot steam
atmosphere for some time. In some cases it may be possible94
for the formation of a thick steam atmosphere (~1-100 bar) to
reflect away enough sunlight to (temporarily) stall the runaway
and stabilize at a surface temperature of 350-550 K (with the
high pressure preventing the ocean surface from evaporating
further) Small, low-gravity waterworlds
may also stall runaway95
by expanding their outer atmospheres as they warm, giving them
more surface area to radiate away heat.
In most cases, though, the surface should continue warming past
water's critical temperature at 647 K, beyond which there is
no longer a distinct liquid ocean; the lower atmosphere and ocean
become a supercritical fluid, with a gradual transition
from gas-like to liquid-like properties at greater pressures. If
the water layer is deep enough, it may transition to odder phases
like an ionic fluid where the water dissociates to
oxygen and hydrogen ions and superionic ice with a
crystal lattice of oxygen through which hydrogen ions travel
freely.
Even small amounts of atmospheric water will form clouds, and these
clouds can significantly increase the albedo of a world. Earth’s overall
albedo is 0.3, even though most of the land and ocean has a lower
albedo, due to water clouds. But that albedo
could conceivably96
vary from 0.25 to 0.5 with fairly minor changes in climate. A thick steam atmosphere could have an albedo as high as 0.8, though
at higher temperatures97
(>1500 K) fewer clouds will form and this will decline (though, per
our discussion of hydrogen atmospheres above, other clouds may become a
factor at these higher temperatures).
Hydrocarbons
Methane (CH4) is a major gas in Titan’s atmosphere
(5.7% near the surface), and trace amounts of
ethane (C2H6) exist there as well. Methane
may have existed in significant amounts in Earth's early atmosphere, but
was mostly removed once atmospheric oxygen appeared.

|
| Image of Titan. NASA |
Methane is commonly outgassed by volcanism under reducing conditions,
but as Earth has aged and the crust has oxidized, this has mostly been
replaced with CO2
outgassing. But more sustained reducing chemistry—such as that of a carbon planet—could produce98
larger amounts of methane, H2, and CO for longer periods. Presuming the planet is small enough that
the H2 is mostly lost, for moderate C/O ratios we should generally expect
CO or CO2 to be the dominant gasses, but at very high C/O ratios, methane and
other hydrocarbons may replace them. As mentioned earlier, the formation
of a silicate crust over a carbide interior may also particularly
encourage outgassing of methane and H2, so it may be possible to see a planet with a broadly Earthlike surface
of silicate rocks and water oceans with large amounts of atmospheric
methane.
Methane can also be produced in large amounts by life by
methanogenesis, either by reaction of existing H2 and CO2 or by breakdown of carbohydrates (produced in turn by
photosynthesis or a variety of other carbon-fixing processes). Similar
abiotic processes99 may be able to react CO2 and acidic water to methane and CO on the surface of some rocks
when exposed to UV light.
A large impact into a planet with a CO2-rich atmosphere, as may have happened on early Earth, could also54
produce a temporary thick atmosphere of H2 and methane that might persist up to a few 10s of millions of years
before these gasses escaped or were sequestered.
There's a whole sequence of hydrocarbons of different carbon chain
lengths (following methane (CH4) and ethane (C2H6) are propane (C3H8), butane (C4H10), pentane (C5H12), etc. as well as variants with more carbon-carbon bonds and fewer
hydrogens), with longer-chain compounds tending to have higher melting and
evaporation points. Hydrocarbons will tend to bond together into longer
chains until they precipitate to the surface as tar. At higher surface
temperatures, longer chains can form before this happens, and lighter
shorter-chain hydrocarbons are more liable to escape (either directly or
by photolysis), so an atmosphere of longer-chain gasses becomes more
likely. But hydrocarbons might break down in various ways as well, and
atmospheric hydrogen in particular
can help hasten this breakdown85
and encourage higher methane levels.
Low amounts of methane can act as a strong greenhouse gas, but in an
atmosphere with significant CO2, a CH4/CO2
ratio greater than 0.1 can cause the formation of a haze that will block
sunlight lower surface temperature100. Lacking CO2, higher levels of methane
can also101
react with nitrogen to form
tholins, a variety of
unstable compounds that form the orange-red haze of Titan. If the methane is produced by either biotic or abiotic surface
reactions that depend on sunlight or are slowed at lower temperatures,
this could create a negative feedback that limits methane to relatively
low levels.
Regardless, though a pure methane atmosphere would appear cyan,
formation of some amount of haze is likely to tint it yellow or brown.
Titan's albedo of around 0.2 may be typical, but it could probably vary
greatly depending on the specifics.
Halogens
Fluorine (F) and Chlorine (Cl) can both exist as gasses at Earthlike temperatures, but are somewhat
rare and tend to react with surface materials to form stable
minerals—though, as mentioned, these will break down at high temperatures. But were we to entertain the idea of seas containing these elements, as
we'll discuss shortly, we might imagine that life could
produce F2 or Cl2 gas in a similar process to production of O2 on Earth. Alternatively, if a world had extremely salty seas, a photosynthetic pathway might
emerge to produce Cl2 from NaCl. Either gas would probably be more prone to sequestration than oxygen,
so such biological production would probably have to be quite vigorous to
build much up. Given more carbon-rich conditions,
various halocarbons like CF4 or CH3Cl could also appear.
Even small amounts of either would tint the sky yellowish-brown. This may
make production by photosynthesis self-limiting, as too much halogen
production might block sunlight.
Sulfur
Sulfur is commonly outgassed as
SO2 or H2S, but tends to ultimately deposit in solid or liquid forms. Small amounts of sulfur can form fairly opaque hazes, though; I've
already mentioned the likely sulfur hazes (mostly S8) of temperate H2 atmospheres, and very high rates of SO2 outgassing
could form similar hazes102 in N2 or CO2 atmospheres. But water vapor will react with SO2 to
produce sulfuric acid (H2SO4),
which will rain to the surface, preventing such hazes103 on any but the driest rocky planets. On Venus84, sulfuric acid forms and rains downwards, but evaporates in the
hot lower atmosphere before reaching the surface, and so remains in
the atmosphere and forms the thick layers of beige clouds, with an
albedo of 0.7.
But at even higher temperatures, surpassing 800 K, water may be
completely lost to escape or sequestration into the interior (made easier
as the hotter surface encourages more melt in the mantle) and sulfide
minerals on the surface may dissolve into a thick, CO2-rich atmosphere, making SO2 and other sulfur gasses more stable (as the usual pathways to
deposition disappear). This is especially true for planets with highly oxidized interiors98 that outgas more SO2. Sufficiently low gravity and high temperature may then cause the
CO2 to escape, leaving heavier SO2
as the primary gas. A similar scenario may perhaps also leave H2S as the primary gas for more reduced planets. But these scenarios have
not received much detailed study, so I'm not confident that all possible
sulfur sinks have been accounted for.
Rock
At temperatures above 1000 K, parts of the rocky crust may begin to
melt and then eventually evaporate, introducing various elements we
don't usually think of as volatiles into the atmosphere.
First off, as temperature approaches 1000 K, the crust will thin and some
surface materials may begin to melt. Excluding giants that can retain
hydrogen or helium, most atmospheres this hot are likely to be dominated
by some combination of CO2, water, N2, SO2, H2S, and hydrocarbons, depending on the sources and sinks present. Presuming
the planet is in close orbit of its star (though the heat from a recent
impact could also form most of the atmospheres we'll discuss), intense
solar radiation may make photolytic O2
and CO more common. Conversely, a planet with a high C/O ratio may lack O2 and CO2
and
form clouds of graphite104, which would then snow down on the surface and form a solid layer.

|
|
Composition of the atmosphere outgassed from a rocky planet with a
surface temperature of 800 K, depending on the internal chemistry
(more oxidized to the right). No losses or photolysis are
accounted for here; presuming that at least
H2 and H2O are lost would make the other gasses proportionally more
common.
Liggins et al. 2022. |
Past 1500 K, the surface will melt more completely and atmospheric escape
will become significant for most of the gasses we've discussed.
Oxygen—both as O2
and smaller amounts of monoatomic O—may remain common as it is released from the breakdown of melting oxide
minerals. Sodium (Na) and potassium (K) will be the first
metals to enter the atmosphere at around this temperature.
Fluorine (F) and chlorine (Cl) will also enter the
atmosphere as their most common minerals break down; if large amounts of
water are still present, significant amounts of HF and HCL
may form105. Clouds of sodium and potassium salts may form and then rain back down
to the molten surface.
If all lighter gasses escape, chlorine and sulfur may remain as the
dominant volatiles, with some continuing input of oxygen; what sort of
atmospheric mixes may result hasn't received much study. But if all
preexisting volatiles completely escapes, the lava ocean will outgas a
thin, sodium-dominated atmosphere.

|
|
Composition of an atmosphere produced above a silicate magma ocean
with no preexisting atmosphere, depending on surface temperature.
Lammer et al. 2022 |
At even higher temperatures, silicates begin to break down and enter the
atmosphere. Past 2000 K, silicon monoxide (SiO) becomes a major
gas, accompanied by small amounts of magnesium (Mg),
iron (Fe), and various metal oxiides. These may
reform silicate minerals106
in the cooler upper atmosphere, forming black or grey clouds with very low
albedos, though they may also be glowing dull red at these high
temperatures. As temperatures increase, the atmosphere becomes thicker
again, passing 0.1 bar at around 3000 K, and titanium (Ti),
calcium (Ca), and aluminum (Al) oxide clouds may
become more prominent. These planets are likely to be tidal-locked, with
the sun-facing side much hotter than the nightside, with corresponding
variations in atmospheric pressure, causing
continuous strong winds107
from dayside to nightside.
But these materials may also escape to space at such high temperatures.
In the most extreme case, a planet may develop a comet-like tail of
escaping rock vapor.
The atmospheric composition may thus shift as different elements are
gradually lost to space completely, the planet effectively evaporating
away over time. Magnesium in particular
may become dominant106
as sodium, iron, and silicon are lost.

|
|
Gasses produced by a lava ocean at 2200 K with a composition
resembling Earth's mantle (top) or continental crust (bottom) as
larger portions of the whole are lost.
Schaefer and Fegley 2009 |
Others
There are a huge number possible minor atmospheric gasses; Earth's
atmosphere alone has about 20 different gasses in the atmosphere at
levels of at least 1 part per billion. But most of these have few sources and many potential sinks so are unlikely to ever
appear as major components of an atmosphere. I'll just go over a couple
other options here to round off this section. Note as well that most of
the liquids I'll mention in the next section could also be atmospheric
gasses on planets above their boiling point.
Noble gasses like neon (Ne) (and helium, which we've
already discussed) are, as you might expect, gasses at most
temperatures, but for that reason tend not to mix into the solid
materials that form non-giant planets and may be easily lost with the
escape of initial hydrogen-rich atmospheres, with little later
outgassing to replace them. Argon (Ar), however, is produced
by the radioactive decay of potassium, and so can be gradually outgassed
from rocky interiors; today it accounts for 1% of Earth's atmosphere.
Also, as with helium and CO, there is perhaps some chance that breakup
of a large white dwarf could form a body composed mostly of neon,
oxygen, and magnesium, with the resulting atmosphere composed of some
mix of neon, O2, and CO.
Ammonia (NH3) and hydrogen cyanide (HCN) are both fairly common cosmically,
but tend to photolyze or oxidize once mixed into planets. We'll discuss
both in more detail in the next section, but in short, neither are likely
to be major atmospheric gasses, perhaps building up to 0.001 bar under
ideal circumstances (for ammonia108, an H2-dominated atmosphere with abundant nitrogen, low UV, and biotic ammonia production; for HCN109, a very high C/O ratio and some nitrogen). But even at such low levels, these and other gasses (like the
aforementioned carbonyls) may help form a variety of prebiotic molecules that later assemble into
life.
Oceans
Liquids necessarily exist in an intermediate range of temperature and
pressure between solid, gas, and supercritical states. A stable body of
liquid on a planet's surface requires some amount of pressure from an
atmosphere, hence why I've put oceans last. But if no atmosphere exists,
the liquid will create its own by evaporating up to the
vapor pressure, though if this gas then escapes to space, continued evaporation of the
ocean can lead to its total loss to space. Preventing such rapid escape
requires a planet of some minimum mass, depending to some extent on the
liquid and the planet's temperature, which is generally high enough that
we'd expect internal pressures of that planet to compress basically any
material to a solid form. So, aside from perhaps very young, molten
bodies, we shouldn't expect to find liquid planets with no solid (or at
least supercritical) core.

|
|
Generalized phase diagram, showing boundaries between the common
phases. Modified from
Matthieumarechal, Wikimedia |
The range of temperatures and pressures required for stable liquids varies greatly, but for our purposes we can generally summarize the extremes with two key points: the triple point, which represents the minimum temperature and pressure at which the liquid can exist (usually; a few materials like water can remain liquid at temperatures below their triple point, but only slightly except at very high pressures); and the critical point, which represents the highest temperature at which liquid can exist and the minimum pressure to remain liquid at that temperature.
Past the critical temperature, supercritical fluids can behave similarly
to liquids at high pressures (though in most cases the pressure really has
to be enormous), but an "ocean" of supercritical fluid would lack a
distinct surface, instead transitioning gradually into the
atmosphere—as discussed a couple times in the previous section. A planet could also conceivably have deep oceans with a liquid surface
that transitions to a supercritical fluid at great depth, but then of
course requires that at least the surface be below the critical
temperature to form a distinct ocean surface, which I'll presume is what
we want in this section.

|
| Based on simple assumptions of surface temperature, regions of stability for oceans of different compounds in orbits of different stars. Ballesteros et al. 2019 |
For your convenience, here are these values for most of the materials
discussed here, as well as I could determine, as well as the phase-change temperatures at sea level pressure on Earth (I left spaces blank where no good information is available—you can usually assume the triple point is pretty close to the 1-atm melting temperature; for simplicity I rounded molar mass to the nearest g/mol for the most common isotopes rather than averaging for Earth's isotope mix as is usually done; several hydrocarbons and HCONH2 have inexact melting/boiling points by their nature; He, CO2, and C sublimate at 1 atm).
|
Material
|
Molar mass
(g/mol)
|
Triple
Point
|
Transitions
at 1 atm (K)
|
Critical
Point
|
|||
|
Kelvin
|
bar
|
melting
|
boiling
|
Kelvin
|
bar
|
||
|
He
|
4
|
2.18
|
0.052
|
4.22
|
-
|
5.20
|
2.27
|
|
H2
|
2
|
13.80
|
0.070
|
13.99
|
20.28
|
32.94
|
12.86
|
|
Ne
|
20
|
24.56
|
0.435
|
24.56
|
27.10
|
44.49
|
27.69
|
|
F2
|
38
|
53.48
|
0.0025
|
53.48
|
85.03
|
144.4
|
51.72
|
|
O2
|
32
|
54.36
|
0.0015
|
54.36
|
90.19
|
154.6
|
50.43
|
|
N2
|
28
|
63.15
|
0.125
|
63.15
|
77.36
|
126.2
|
33.90
|
|
CO
|
28
|
68.10
|
0.154
|
68.13
|
81.6
|
133.2
|
34.98
|
|
Ar
|
40
|
83.81
|
0.689
|
83.81
|
87.30
|
150.7
|
48.63
|
|
C3H8
|
44
|
85.47
|
10-9
|
85.5
|
~231
|
369.5
|
42.49
|
|
SiH4
|
32
|
88.48
|
0.0002
|
88.1
|
161.2
|
269.2
|
48.45
|
|
CF4
|
88
|
89.4
|
0.001
|
89.5
|
145.3
|
227.5
|
37.45
|
|
C2H6
|
30
|
89.89
|
0.00001
|
90.4
|
184.6
|
305.3
|
48.72
|
|
CH4
|
16
|
90.68
|
0.117
|
90.69
|
111.6
|
190.6
|
45.99
|
|
Kr
|
84
|
115.8
|
0.735
|
115.8
|
119.9
|
209.5
|
55.25
|
|
OCS
|
60
|
134.3
|
|
134.3
|
223.0
|
378.8
|
63.49
|
|
C4H10
|
58
|
134.6
|
7*10-6
|
~136
|
~273
|
425.1
|
37.96
|
|
PH3
|
34
|
139.4
|
|
140.3
|
185.5
|
324.5
|
65.37
|
|
C5H12
|
72
|
143.46
|
7*10-7
|
~143
|
~309
|
469.8
|
33.60
|
|
C2H6O
|
46
|
150
|
4*10-9
|
159.0
|
351.4
|
514
|
63
|
|
HCl
|
36
|
159.0
|
0.139
|
158.9
|
188.1
|
324.7
|
82.56
|
|
CS2
|
76
|
161.1
|
|
161.5
|
319.4
|
552
|
79
|
|
Xe
|
131
|
161.4
|
0.818
|
161.4
|
165.1
|
289.7
|
58.42
|
|
Cl2
|
70
|
172.1
|
0.014
|
171.6
|
239.1
|
416.9
|
79.91
|
|
CH3Cl
|
50
|
175.4
|
0.0087
|
175.8
|
249.3
|
416
|
67.14
|
|
CH4O
|
32
|
175.5
|
0.0019
|
175.6
|
337.8
|
513
|
79.5
|
|
CH2O
|
30
|
|
|
181
|
254
|
|
|
|
N2O
|
44
|
182.3
|
0.879
|
182.3
|
184.7
|
309.5
|
72.40
|
|
H2S
|
34
|
187.6
|
0.233
|
187.7
|
213.6
|
373.3
|
89.7
|
|
HF
|
20
|
189.8
|
|
189.6
|
292.6
|
461
|
65
|
|
NH3
|
17
|
195.4
|
0.061
|
195.4
|
239.8
|
405.5
|
112.8
|
|
SO2
|
64
|
197.7
|
0.017
|
201
|
263
|
430.8
|
78.84
|
|
CO2
|
44
|
216.6
|
0.517
|
194.7
|
-
|
304.2
|
73.80
|
|
HNO3
|
63
|
|
|
231
|
356
|
648.5
|
80.58
|
|
Fe(CO)5
|
196
|
|
|
252.2
|
376
|
|
|
|
HCN
|
27
|
259.9
|
|
259.9
|
299
|
456.9
|
53.20
|
|
N2O3
|
76
|
|
|
261.9
|
294.8
|
630.5
|
66.75
|
|
NO2
|
46
|
|
|
263.8
|
294.3
|
431.4
|
10.13
|
|
H2O2
|
24
|
|
|
272.7
|
423.3
|
728
|
220
|
|
H2O
|
18
|
273.2
|
0.0061
|
273.2
|
373.2
|
647.1
|
220.6
|
|
N2H4
|
32
|
274.6
|
|
275
|
387
|
653
|
147
|
|
HCONH2
|
45
|
275.6
|
|
~275
|
483
|
|
|
|
HC3N
|
51
|
|
|
278
|
315.6
|
|
|
|
H2SO4
|
98
|
|
|
283.5
|
610
|
927
|
460
|
|
H3PO4
|
98
|
|
|
315.5
|
|
|
|
|
K
|
39
|
336.35
|
|
336.7
|
1031
|
2223
|
160
|
|
Na
|
23
|
|
|
370.9
|
1156
|
2573
|
350
|
|
S8
|
256
|
388.3
|
0.00003
|
388.4
|
717.8
|
1314
|
207
|
|
Mg
|
24
|
922
|
|
923
|
1363
|
4100
|
|
|
NaCl
|
58
|
1074
|
0.30
|
1074
|
1738
|
3900
|
260
|
|
FeS2
|
120
|
|
|
1180
|
|
|
|
|
Fe
|
56
|
|
|
1811
|
3134
|
8500
|
|
|
SiO
|
44
|
|
|
1975
|
2150
|
|
|
|
SiO2
|
60
|
|
|
1986
|
3220
|
|
|
|
TiO2
|
80
|
|
|
2116
|
3245
|
|
|
|
Al2O3
|
102
|
|
|
2345
|
3250
|
|
|
|
CaO
|
56
|
|
|
2886
|
3120
|
|
|
|
MgO
|
30
|
|
|
3125
|
3870
|
|
|
|
C
|
12
|
4500
|
101
|
3915
|
-
|
|
|
Most simple liquids are colorless, but I'll note some exceptions, and
they could conceivably be colored by any number of dissolved
impurities.
Water
Of course. There’s some debate about exactly how Earth received all its
water—in particular whether it was part of the original material that
formed the planet or it was delivered later by comets—but however it
arrived, some amount of water seems to have made it into every planet
and major moon in the solar system, so it doesn’t appear hard to come
by. Earth’s water regularly cycles in and out of the interior, such that
may be several more ocean’s worth of water stored in the mantle (don't
picture giant aquifers; it all exists either mixed into magma or as
individual molecules within the crystal structures of certain minerals).
Thus, we could probably afford to entirely lose the oceans a couple
times over before the planet became completely dry.
Recent research110
indicates that (at least in red dwarf systems) most planets of Earthlike mass are probably either relatively dry like Earth, which is under 0.05%
water by mass even including mantle water, or very wet, with a roughly 50%
water, and there are few—but not no—planets in between.
More recent research111
has suggested that many exoplanets identified as small gas giants may be
hydrogen-ocean or hycean worlds, with a deep water ocean below
a thick H/He atmosphere.
Within the "dry" group, feedbacks between surface oceans and the mantle should tend112
to prevent the surface from being completely inundated while less than
0.2% of the planet’s mass is water, and
also tend113
to reduce the size of these oceans as the mantle ages and cools; Earth 3
billion years ago may have had twice the amount of water in its oceans,
and given another 3 billion years the oceans may drop to 1/4 of their
current volume were they not likely to boil away first due to the warming
sun. So we can mostly expect water oceans to either be partial and
(relatively) shallow or global and very deep.
Once a global ocean114
gets deep enough—around 150 km deep for an Earth-mass planet with a
temperate surface—the pressure at the ocean’s base will be so intense as to force water at
the bottom into unusual, dense phases of ice which remain solid to high
temperatures. Initially this ice layer is only a few kilometers thick, and heat from
the interior can get the base of the ice hot enough to melt again and form
a secondary liquid ocean below it. But as the ocean grows deeper, the ice
layer thickens and the lower ocean thins. Past around 230 km, the ice
layer grows to over 50 km thick and liquid water below it exists only
intermittently in a thin layer.

|
| Varieties of waterworld ocean structures, with increasing total depth to the right (depth not to scale). Noack et al. 2016 |
A world with under half Earth's surface gravity and a surface
temperature of 400 K
could get115
liquid oceans over 1500 km deep, but such a hot surface would require a
thick atmosphere (~10 bar) to stop it boiling—or we could let it boil to produce a thick steam atmosphere, but that
could lead into the runaway greenhouse scenario we discussed earlier. An
even hotter surface could also cause an even deeper layer of supercritical
water to form under the liquid ocean.

|
|
Possible internal structures for a planet 8 times Earth's mass
composed of 70% water, depending on the pressure and temperature
at the surface or top of atmosphere.
Nixon and Madhusudhan 2021 |
On the other end of the scale, dry, cold worlds below water's usual
melting point could have small bodies of mineral-rich brines. In
the most extreme case116, a lithium-ammonia-rich brine could remain liquid to as little as 90
K.
Open water has a very low albedo of 0.06, but of course water oceans
are likely to be accompanied by ice and clouds with much higher albedos.
Liquid water is actually blue on its own independent of the color of the
atmosphere, but various minor impurities could conceivably alter its
color—green being common on some areas of Earth due to the presence of
plankton, but just as alien vegetation could be many different colors,
alien microbes could color their seas many hues. Earth's oceans may have
been tinted several other colors in the past:
- Earth's early, oxygen-poor oceans may have contained117 significant amounts of ferrous-ferric hydroxy salts, a.k.a. green rust, potentially tinting them green long before green algae appeared.
- When oxygen was first produced 2.5 billion years ago, it would have converted dissolved iron to the more familiar iron oxide rust, tinting the oceans red for a period until enough oxygen accumulated to remove iron from the oceans.
- Even after oxygen appeared, there may have been an extended period, perhaps up to ~1 billion years ago, when the oceans remained relatively oxygen-poor and sulfur-rich, tinting areas of the ocean118 milky white or turquoise with sulfur and perhaps including black patches of bacterial mats and organic debris welling up from the ocean floor near coastlines. It also would have smelled awful.
- Before around 700 million years ago, the dominant photosynthetic life in the oceans may have been119 red or purple algae, tinting large areas pink.
Hydrocarbons
The only other surface liquid in the current solar system is the hydrocarbon lakes of Titan. Titan has a full “methane cycle”
analogous to Earth’s water cycle, with clouds, rain, rivers, and seas.
The seas themselves are mainly restricted to the poles and
are likely composed120
of a mix of 3/4 ethane (C2H6), 10% methane (CH4), 7%
propane (C3H8), and smaller amounts of
butane (C4H10), hydrogen cyanide (HCN), nitrogen (N2), and Argon (Ar). Unlike Earth's water, there is
far more methane121
within the atmosphere than in the seas, though large amounts
may also exist in the crust122
as what we might call "groundmethane", like our groundwater.
These seas can form because Titan is cold enough to lock all its
water into the ice crust; a warmer Titan would have water-ammonia
oceans instead. But a carbon planet could lack water and have
hydrocarbon seas up to higher temperatures. Much as we discussed, the
methane and ethane might evaporate but may then bond into larger
molecules, forming a more viscous sea of tar. What sort of climate cycles such a world might have has not
received much formal research.
The variety of hydrocarbons and the even broader variety of organic
molecules they can form when interacting with the atmosphere and surface
may make for a lot of chemical complexity. Rain and snow may consist not
just of hydrocarbons but also HCN, cyanoacetylene (HC3N), and more complex tholins—all of which have various melting temperatures, so mixed rain and snow
of different compounds may be the norm. The snow
may form123
a thin film on the surface of the oceans, damping waves and slowing
evaporation.
Sulfur
There are a number of sulfur compounds that exist in liquid form in
small amounts throughout the solar system and could conceivably gather
into more stable lakes or oceans under slightly different
circumstances.
First off, Venus has clouds and rain of sulfuric acid (H2SO4) in its upper atmosphere, formed from water and volcanic sulfur
compounds. Though the temperature and pressure in the lower atmosphere
are strictly within the liquid range for
H2SO4, it nonetheless tends to dissociate in the heat to water and
SO3, which evaporate before reaching the ground. Were Venus cooler (below
around 550-600 K), the
H2SO4
might instead pool on the surface. It might then react with the surface
and deposit as various minerals, but sustained volcanism could perhaps
supply enough sulfur to eventually saturate these sinks, eventually
forming lakes or oceans of
H2SO4. Even on temperature worlds, high levels of sulfuric volcanism could
introduce some
H2SO4
into water oceans, and various mixes of water and
H2SO4
could conceivably exist over a broad temperature range.
Under more reducing or water-poor conditions, atmospheric sulfur
compounds
might instead124
form pure sulfur (mostly S8), which could remain liquid above around 388 K. Io appears to form
temporary lakes of molten sulfur (with frozen surfaces) warmed by
volcanic activity. Sulfur has a very high critical temperature of 1318
K, and so might form some of the warmest oceans of any typical volatile
before temperatures get high enough to melt the whole crust. At
temperatures above ~800 K, sulfide minerals like pyrite (FeS2) may decompose to produce sulfur, which should remain liquid at
pressures above a few bar.
And at colder temperatures, volcanic sulfur dioxide (SO2) or hydrogen sulfide (H2S) might themselves form seas, though the latter has a narrow range of
liquid temperatures. Again, such liquids might exist in pockets below
the surface of Io. At a high C/O ratio, carbon disulfide (CS2) or carbonyl sulfide (OCS) might be possible, but I've never
seen more than a passing reference to the idea in the literature.
Various mixes of these liquids could also form, so there is overall a
fairly broad range of sulfur seas possible if enough sulfur can be
brought to the surface. The appearance could vary greatly:
H2SO4
and
SO2
are clear but might be tinted by various dissolved minerals; Molten
sulfur would vary between transparent yellow at low temperatures, red
between around 430 and 470 K, and black at higher temperatures; CS2
might appear yellow or red.
Ammonia
Ammonia (NH3) is fairly common in ice in the outer system, and as mentioned may be
the ultimate source for nitrogen atmospheres. However, under UV it tends
to photolyze to N2 and H2, and getting those gasses back to NH3 is far more difficult. But UV photolysis should be less of a problem for planets orbiting
redder stars or protected by thick atmospheres or surface ice, and
at least one recent study125
has suggested that the right mix of hydrogen and nitrogen may make
ammonia more stable.
The next problem is that ammonia and water ices are often mixed
together, with water tending to be more common, and there doesn't seem
to be any good way to remove the water and leave the ammonia. Ammonia
does have a lower freezing temperature than water, but water/ammonia
mixes have a lower freezing temperature than either. Were a water-heavy
mix of the liquids to be cooled, the water would freeze first, leaving
the remaining liquid ammonia-enriched,
up to a maximum126
of about 33% ammonia at 176 K; any further cooling would then freeze the
whole remaining mix. Thus, we are probably more likely to encounter
mixed water/ammonia seas than pure ammonia, and such seas are suspected
to exist below the ice of some outer moons like Titan.
On its own, ammonia is clear, but dissolved alkali metals might tint an
ammonia ocean brown.
Carbon Dioxide
CO2 is
not usually thought of as likely to form seas due to its high triple
point pressure; a surface pressure of at least 5.18 bar is required
for liquid CO2 to form. If a planet has volcanic CO2 outgassing which outpaces any sequestration (requiring either
a dry surface, low sunlight, or very high rates of volcanism) then
this isn't that hard to achieve, as we can see on Venus, but then
the greenhouse heating may raise the surface temperature
beyond CO2's critical point at 304 K.
But for planets with less sunlight127 (beyond about 1.5 AU from a sunlike star) such that temperatures remain lower, CO2 clouds can form as CO2 levels increase, cooling the planets by raising its albedo
and eventually overcoming the greenhouse effect and causing
temperatures to drop with further increases in CO2. Lower temperatures tend to slow sequestration, so the CO2 should continue to accumulate until the surface is cold
enough for it to rain out, forming oceans on the surface. This removes
excess CO2 from the atmosphere, preventing further cooling and
stabilizing the climate. Planets near the inner boundary of this
region could also oscillate between cool states with CO2
oceans and warm states where they boil off due to destabilizing
climate feedbacks.
Mixed water-CO2 oceans could also form this way, though if temperature drops
below 283 K (not far below CO2's critical point), the CO2 would instead become trapped in clathrates, water ice with trapped gas, which would accumulate on the floor
of the water ocean.
I can't say much about how these oceans would appear (liquid CO2 itself is clear), but given the very thick, cloudy
atmospheres formed in this process (around 20 bar), these worlds
might be pretty dark.
Nitrogen
Nitrogen (N2) ice is common on some small bodies of the outer solar; if any of these
were larger and had a substantial atmosphere (above 0.13 bar and 63 K),
they might conceivably allow for the ice to melt, while other volatiles
remain frozen. Pockets of liquid N2 are believed by some32
to exist below the surface of Triton.
Hydrogen
Hydrogen (H2) is an obvious candidate, given how common it is as a gas. The main
issue is that its critical point is a mere 33 K; even without any solar
heating, an Earthlike planet with a hydrogen atmosphere will likely
retain a higher surface temperature than that just through retention of
geothermal heat, and larger planets more likely to have atmospheric
hydrogen will tend to retain even more internal heat. Still, geothermal
heating declines with age and it is just about conceivable that there
may be old rogue planets out there (orbiting no star) that are both
large enough to have formed with a hydrogen-rich atmosphere and small
enough to have cooled below its boiling point, allowing hydrogen seas to
form.
Hydrogen Cyanide
Another material that's fairly common in the cosmos but often tends
to break down once mixed into planets, though Titan has some in its
lakes and clouds. It should be reasonably stable under strongly
reducing conditions, and can be produced by UV light, lightning, or
impacts, especially109 at high C/O ratios. For such conditions to form large amounts of HCN
but not larger amounts of water or hydrocarbons seems unlikely, but
might be worth considering nonetheless; HCN is a tad heavier than
water and methane, so could conceivably remain if they escaped to
space.
Alcohol
Simple alcohols like methanol (CH3OH) and
ethanol (C2H5OH) are fairly common in comets and the interstellar
medium. They're rarer on planetary bodies in the solar system,
though could perhaps have been present on the early Earth, and
short-lived methanol seas
have been proposed128 for early Mars.
Though initial delivery of alcohols by comets is likely to be diluted
in much larger amounts of water or methane, sunlight-aided oxidation
of methane with oxygen or hydrogen peroxide (H2O2) could
produce more methanol,
especially129 if helped along by
iron or copper catalysts. A methanol/water mix is also possible; in an
initially water-heavy mix, the water would freeze out first at low
temperatures, and unlike ammonia the remaining liquid would become
methanol-dominated—up to130 88% before completely
freezing at 157 K.
The main issue is that the same materials that oxidize methane to
methanol would also tend to further oxidize methanol to and water.
Preventing this likely requires either a very well-timed shift in
surface chemistry or geochemical processes that add methane and oxygen
and remove water.
Formamide
Formamide (HCONH2) is a fairly uncommon
volatile in the cosmos but easily formed by the reaction of water and
HCN, or by lightning or impact events in an atmosphere with a mix of
the CHON elements, and likely did form in at least small amounts on
the young Earth. As it has a higher boiling point than water (483
K at 1 bar), a pond of mixed water and formamide in a hot, dry
environment will tend to lose water until only the formamide remains.
But in a wetter environment, the formamide will break down again, and
an oxidizing atmosphere will inhibit the formation of cyanide; so
formamide no longer forms in large amounts on Earth, but perhaps a
dryer world with a reducing atmosphere could form persistent lakes or
seas of formamide. Various mixes of formamide with water, HCN, or
hydrocarbons could also be possible.
These possibilities are intriguing because liquid formamide provides
an excellent environment for the formation of complex organic
molecules.
Some researchers131 have even proposed
that life on Earth may have begun in isolated formamide ponds and then
only later spread into the water oceans.
Halogens
Fluorine (F) and chlorine (Cl) (part of the group of halogen elements) are not terribly common, but they are somewhat concentrated in Earth's crust, forming a variety of minerals, and it is conceivable that they might be even more concentrated on another planet, with volcanic activity thus producing some amount of HF or HCl. Usually we'd expect such conditions to also produce larger amounts of water, but perhaps if the water escaped into space or was locked in the mantle132, the halogens that remained in the crust could then produce HF or HCl seas through later volcanism.
Initial seas of sulfuric acid might also help release halogens from the
crust, forming mixed oceans; perhaps the sulfur could later be
preferentially sequestered, but that's wild speculation on my part.
Remember as well that on planets heated to over 1000 K, HCl and SO2
might become the dominant volatiles if all lighter gasses escaped; perhaps
later cooling could then allow the HCl to rain back down and form seas
(though avoiding deposition of the chlorine in salts may be difficult). A
large impact event that temporarily melted the crust
might provide133
the ideal circumstances for this intense heating and then cooling.
Under more carbon-rich conditions, these processes might also produce
halocarbons (CF4, CHCl3), which much like regular hydrocarbons can assemble into larger
molecules.
None of these scenarios seem especially likely or have been explored in much depth, but they are tantalizing as HF and HCl are similar to water in many ways but abundant halogens could allow for intriguing new forms of biochemistry, which we'll discuss in a later post.
Lava
Much as we discussed in the atmospheres section, many materials we
don't usually think of as forming oceans might do so if the surface gets
hot enough. Essentially all substantial planets should pass through a
molten stage as they form, and later large impacts could also briefly
melt at least part of the surface. How long these temporary lava seas
last
depends strongly134
on how thick of an atmosphere forms from gasses released from the rock
as it melts; if the rock is relatively "dry" and the atmosphere remains
thin, the surface will cool and solidify within a few thousand years;
but if a thick steam or
CO2
atmosphere forms, this could help trap heat and keep the surface molten
for 10s of million of years. If a thick steam atmosphere forms and the
planet is relatively close to its star (within ~0.8 AU from a sunlike
star), the runaway greenhouse heating
may keep the surface molten136
for as long as outgassing of water from the lava ocean can replace
atmospheric losses; potentially billions of years for a body with 10 or
more times Earth's water content.

|
|
Time of survival for a global lava ocean for an Earthlike planet
with an albedo of 0.2 orbiting a sunlike star based on orbital
distance and water content (1 MEO
being equivalent to Earth's oceans).
Hamano et al. 2015 |
Internal sources of heat from radioisotope decay or tidal heating may
make it easier for the surface to melt but are unlikely to keep it
molten on their own for long; radioisotope concentrations would need to
be absurdly high to produce enough heat (some short-lived isotopes like
aluminum-26 might help keep planets molten for longer when
they first form but will then near completely decay away within a few
million years) and tidal forces strong enough to induce that much
heating should also quickly change the planet's orbit or rotation to
reduce that heating.
The longest-lived lava oceans will form on planets close enough to
their stars to be heated above their boiling point regardless of the
presence of an atmosphere. Any such planet will form a rock vapor
atmosphere and likely experience atmospheric escape, such that the whole
planet will essentially evaporate away given time;
this might take136, around a billion years for an Earth-mass planet heated to over 2000 K,
but could be a good deal longer for more massive planets.
As mentioned, sulfide minerals may decompose at around 800 K, potentially
producing molten sulfur. Alkali salts like NaCl will melt at
1000-1500 K and could perhaps pool on the surface if the planet retains a
thick enough atmosphere to keep them liquid. The silicates that
make up the bulk of rock will mostly melt at 1500-2000 K. As these
materials melt, they'll also vaporize and escape to space, leaving the
lava more enriched with less volatile materials like calcium and aluminum oxide (CaO and Al2O3) which may then solidify again, but even these will melt at ~2200 K.
More iron-rich lavas may be possible if the rocky mantle has
been mostly removed before the surface melts, but otherwise iron tends to
more readily vaporize and escape to space than other metals.
Any planet orbiting its star close enough to be heated to such extremes
is likely to be tidal-locked to it. Presuming any preexisting volatile
atmosphere has been lost, if the dayside temperature remains below 3500 K
then the rock vapor atmosphere will be quite thin and it and the viscous
lava ocean will transport heat poorly. For a dayside peak temperature of 2500 K, the nightside could remain137 as cold as 50 K.

|
| Concept of CoRoT-7b. Leger et al. 2011 |
The result is what we might call a "lava eyeball world":
The dayside would be dominated by a circular lava ocean, with a
shoreline where the surface temperature drops below its melting
point. There would be a fairly broad
partially molten region138
along the shore, probably with a thin solid crust; perhaps
convection currents in the ocean could pull off sections of rock
just as icebergs calve off of glaciers into our oceans.
Rock vapor will evaporate off the magma ocean and deposit on
the nightside crust, forming a pressure gradient and thus
prevailing winds towards the nightside with windspeeds as high
as kilometers per second (though that's less dramatic than it
sounds in such a thin atmosphere). Metals will crystallize and
snow out of the vapor at different temperatures, forming
concentric rings of solid metals or metal oxides on the crust
around the ocean's shores.
Finally, the nightside will be cold enough that an icecap of
water or other volatiles could conceivably form, bizarre as that
may sound under the circumstances. Before you ask, air pressure is too low for surface liquid
water to exist in the transition zone; the silicate atmosphere
freezes out before reaching the nightside, and any more volatile
gasses would rapidly escape on reaching the dayside, so in
effect the nightside has no atmosphere. But local water aquifers
under the ice are a possibility, and so life may be as
well.
Others
In addition to those liquids mentioned, there are a number of other
liquids that have been suggested at one point or another as possibly
forming lakes or seas, but have received almost no formal study.
Carbon monoxide (CO) is a fairly common ice on very cold bodies,
and has a similar triple point to nitrogen (0.15 bar and 68 K), so should
similarly be a possible liquid on larger cold bodies than we see in the
solar system, though may be restricted to more reducing conditions.
Perhaps N2-CO mixes could even occur.
While we're at it, oxygen (O2) also has a similar but somewhat more permissive triple point (0.0015
bar and 54 K), but of course O2's reactivity makes it rare; it could be a tad more stable at low
temperatures, but producing large amounts of it might also be more
difficult in such conditions (as we wouldn't expect much in the way of
vigorous photochemistry or biological activity). Some icy bodies have very
thin oxygen atmospheres due to photolysis of surface ice and then hydrogen
escape, so perhaps that could condense if it managed to build up to a
high-enough pressure.
Noble gasses also become liquids at sufficiently low temperatures:
helium's (He) critical point of 5.2 K is probably simply too cold
for any substantial body to have reached within the current age of the
universe, but those of neon (Ne; 45 K) and argon (Ar; 151 K) are more
reasonable. As mentioned before, the issue is that these are unlikely to
be common on planet surfaces, but there is some argon in Titan's seas, and
again we could imagine breakup of an oxygen/neon/magnesium white dwarf
forming a body with a neon-rich surface (much as for hydrogen, such a body
would probably have to be isolated from solar heating and quite old to get
colder than neon's critical point).
Metal carbonyls like iron pentacarbonyl (Fe(CO)5)
can form naturally140
under very reducing and metal-rich conditions and are liquids at similar
temperatures and pressures to water and other volatiles, so could perhaps
become common under one of the scenarios for formation of a metal-rich
surface we discussed earlier. Color would vary, with Fe(CO)5 tending to be rusty orange.
Hydrogen peroxide (H2O2) is usually highly reactive, but could perhaps replace water in very
oxidizing conditions. A water-H2O2
mix could be
an intriguing solvent141
for life in cold, dry conditions like the modern surface of Mars, though
pure H2O2
might be too oxidizing for life.
Hydrazine (N2H4)
has similarly been discussed129
as an intriguing potential solvent for alien life from a biochemical
standpoint, but always with the conclusion that natural bodies of
hydrazine are difficult to justify geochemically given how reactive it
is.
Nitric acid (HNO3) and various nitrogen oxides (NO2, N2O3, etc.)—which will react with water to form HNO3—are liquids
at decently broad temperature ranges and form in small amounts in a
variety of conditions, but are fairly reactive and would likely be stable
only in very oxidizing conditions, and even then there doesn't seem to be
a good scenario for them to form in the absence of much larger amounts of
other volatiles. NO2 is
reddish brown and HNO3 is clear
but would likely be tinted yellow or brown by small amounts of NO2.
Phosphine (PH3) and phosphoric acid (H3PO4) are liquids over fairly broad ranges of temperatures, but rare due
to phosphorus's tendency to form stable phosphate materials. But
phosphorus bonds more weakly to carbon, so a very high C/O ratio could
perhaps make them more likely.
Silane (SiH4) is liquid under similar conditions to nitrogen, and the two actually
mix together pretty well, which may have
intriguing implications142
for the possibility of exotic silicon-based life (though there are still a
number of issues we'll discuss at a later time). But silicon has a strong
tendency to form stable solids with both oxygen and carbon, and it would
be hard to imagine a scenario where both were absent such that large
amounts of silane could form.
Alien Skies
Perhaps the most popular and effective way to establish a fictional world as alien and interesting is to have the characters look up, so it would be remiss of me not to address what they might see in our constructed systems. I’ve already discussed the color of the atmosphere; what effect this has on the color of objects seen through the atmosphere depends on exactly why the atmosphere is that color. If the sky is colored due to scattering, that color will be subtracted from light passing through it; the sun appears yellow from Earth’s surface rather than its true white because of the scattering away of blue light. If the sky is colored by particulate matter, this color will be added; any object seen from Mars’s surface will be tinged red (scattered light from one object can also tinge other objects to some extent, if they're far dimmer than the primary light source in the sky).
By way of example, friend of the blog Luke Campbell has done
some admirable work
modelling the color of skies with varying atmospheric pressure, stellar
spectra, and composition; Teacup Ae orbits a K5 star and I'll give it a
thicker atmosphere than Earth, so based on these results we can expect a
generally paler sky with a slight orange tinge at the horizon that then
becomes much more prominent at twilight.
The direction to an object in the sky depends on an interplay of
distance, inclination, obliquity, latitude, time of day, and time of year
that I won’t dig into here. But just as a point of reference,
here’s the formula144
for the maximum angle between the horizon and a satellite—be it a moon,
ring system, or whatever else—that has a circular, equatorial orbit:
I find it super amusing that carbon planets sound so valuable to us now,
but by the time we have the technology to get to them both diamonds and
oil will probably cost peanuts to artificially produce.
Past that, we can summarize most of what we want to know with two
values, the
apparent diameter and the
apparent magnitude; how big
and bright is it in the sky?
The apparent diameter of a spherical body is straightforward
trigonometry:
δ
= apparent diameter
r
= radius of spherical object (any unit so long as
D is the same)
D
= distance from observer to center of spherical object
For small angles, sin-1(r/D) will be pretty close to (r/D) anyway (within about 1% error where
r/D is less than 1/4), so you can skip the trigonometry in a hurry; so,
for example, were the moon 2 times its current distance from the Earth
but had 3 times the radius, we would expect it to appear about 3/2 as
wide in the sky as it currently does.
This incidentally gives you some idea of when eclipses might occur:
for, say, a planet's moon to eclipse its star requires that the moon's
apparent diameter from the planet's surface be larger than the star's.
Apparent magnitude can be a bit trickier. For a star, an
absolute magnitude that is
independent of the distance from the observer can be calculated based on
luminosity:
M
= absolute magnitude
L
= luminosity (relative to sun)
Note that magnitudes are logarithmic, to match human perception of
light; we perceive large changes of luminosity in bright conditions to
be equivalent to small changes in dim conditions. This allows us to see
details at night without being overwhelmed by excess information in the
day, and we should probably expect it to be a trait shared with any
organism that has sight as a dominant sense. Note also that brighter
objects have lower magnitudes for…historical reasons? I’m honestly not
sure. So a decrease of magnitude by 1 corresponds to an increase in
luminosity by a factor of ~2.5.
And finally, note that this is a
bolometric absolute
magnitude, meaning that it accounts for all light across the spectrum.
To compare how stars other than the sun would appear to human eyes, we
have to apply a
bolometric correction factor. Unfortunately there’s no easy, simple formula to estimate this
factor, but
extensive tables143
exist for observed factors for given effective temperatures.
This correction is specific to the spectral range of human vision, of
course, but there’s some justification for thinking that an alien
organism with a similar natural lifestyle might have a similar visual
range, regardless of the star it lives near—more on that when we discuss
biology.
Once an absolute magnitude is known—bolometric or visual—the apparent
magnitude can be calculated for an observer at a given distance:
m
= apparent magnitude
M
= absolute magnitude
D
= distance to observer (parsecs; 1 psc = 3.261 ly = 2.063*105
AU = 3.086*1013 km)
For planets (or other similar bodies) the process is similar, starting
with a
planetary absolute magnitude
that indicates the reflection of light by a planet orbiting a given star
independent of the planet’s distance from that star or the observer’s
distance from the planet—but is not on the same scale as stellar
absolute magnitude.
H
= planetary absolute magnitude
M
= star absolute magnitude (4.74 for the sun)
r
= planet radius (Earth radii)
p
= geometric albedo.
The geometric albedo used
here, which represents the light reflected by a planet directly back at
the light source, is different from the
bond albedo used for the
effective temperature calculation before. The two are typically similar
but the geometric albedo can be higher or lower than the bond albedo,
and for solar system bodies it is usually the former, with airless
bodies tending to have the greatest difference. Earth has a bond albedo
of 0.3 but a geometric albedo of 0.43.
That in mind, the apparent magnitude of the planet—which is on the same
scale as that of stars—can then be approximated based on the relative
positions of the star, planet, and observer:
m
= apparent magnitude
H
= planetary absolute magnitude
DS
= distance from star to planet (AU)
DO
= distance from observer to planet (AU)
α
= phase angle (degrees); angle between the lines connecting the center
of the planet to the star and to the observer.

|
| Modified from Renerpho, Wikimedia |
This is a rough approximation, but thanks to the logarithmic scale
small errors shouldn’t change the results too much. It also assumes that
the bodies are fairly far apart, such that the observer can see most of
one hemisphere, and the planet produces no light of its own (if it
does, you can use the same magnitude formulas as for stars for this emitted
light, and, thanks to the logarithmic scales here, we can assume the light
of lower magnitude dominates over the other).
By way of comparison, here are the maximum apparent diameters and
magnitudes of several objects as seen from Earth (for Venus, the two
maxima are achieved at different times):
|
Body
|
Sun
|
Moon
|
ISS
|
Venus
|
Sirius
|
|
Apparent Diameter (°)
|
0.54
|
0.57
|
0.017
|
0.018
|
1.6*10-6
|
|
Apparent Magnitude
|
-26.74
|
-12.90
|
-5.90
|
-4.92
|
-1.47
|
6.5 apparent magnitude is the approximate limit for detection by human
eyes on a clear, moonless night, though if all other stars or sources of
light were removed there is no specific limit—the human eye is somewhat
sensitive even to individual photons, though we probably can't expect to
see extremely dim objects with any detail. The Hubble Space Telescope is
sensitive to 31.5 magnitude. There is no such limit for apparent
diameter; even a point source of light is visible if it is bright
enough. But 0.02° is about the limit to perceive an object as anything
other than a point.
So far as I can tell, the largest apparent diameter of any planet
viewed from any moon in the solar system is Jupiter as viewed from
Metis, at 67° (as mentioned, my apparent magnitude formulas aren’t valid
this close, but I’d estimate it to be in the neighborhood of -21 at
maximum). Viewed from a flat area on Metis facing Jupiter, the planet
would stretch over 1/3 of the distance from horizon to horizon, which
means the moon spends 1/6 of its orbit in Jupiter’s shadow. However, for
most major moons the planets are surprisingly small in the sky; from
Ganymede, Jupiter appears 7.65° in diameter (smaller than the palm of
your outstretched hand) and about -16 in magnitude; from Titan, Saturn
appears 5.46° in diameter (16.85° including the rings) and -14 in
magnitude. They’d certainly make for impressive sights, but not loom
across the sky quite as much as sometimes depicted.
But that’s just our system. As a general rule, planets in the habitable
zone of stars smaller than the sun will observe their stars to be larger
but dimmer in visual light compared to the sun as seen from Earth. In
the tightly-packed TRAPPIST-1 system, many of the planets are of similar
size to Earth but much closer together. From TRAPPIST-1f—the most likely
to be habitable (based purely on its orbit)—the star appears 1.67° in
diameter and -20.2 in magnitude (by visual light). The nearby planet
TRAPPIST-1e appears 0.48° in diameter at closest approach, almost as
large as our own moon. If TRAPPIST-1e were replaced with a Neptune-sized
planet, it could actually eclipse the star as seen from TRAPPIST-1f. A
larger planet might destabilize that particular system, but at any rate
it appears feasible for such interplanetary eclipses to occur in tight
systems.

|
| Concept of the system Gliese 581. ESO |
θ
= maximum angle between horizon and satellite
L
= latitude of observer
a
= satellite semimajor axis (any unit so long as
r is the same)
r
= radius of planet
As a final note, we may want to talk about how light sources in the sky
affect ambient light conditions on a planet’s surface; i.e., how
“well-lit” the ground will appear. For this, we can return to measures
on insolation. At the equator at midday during the equinox, the sun is
delivering about 1360 W/m2 to the top of the atmosphere and
on a clear day 1050 W/m2 will make it to the surface without
being scattered away, but if we include light that is scattered but
still makes it to the surface then we can raise that value to 1120
W/m2.
That’s for a surface directly facing the sun. As a surface is angled to
the sun, the light is spread over a greater area, reducing the
insolation at any one spot (the sunlight also has to pass through more
of the atmosphere, increasing the amount scattered). Walk just 30° north
or south of the equator—or sit still and wait for 2 hours—and insolation
will be halved, even though to our eyes it still appears perfectly
bright. Again, this comes back to the logarithmic nature of our
perception of light (and adjustment of sensitivity by dilating and
contracting the pupil). An overcast day can have less than 1/10 the
insolation of a sunny day and still appear decently lit.
We could describe all light delivered in terms of power per area like
this, but by convention it’s often described in terms of
illuminance, which is measured in lux, a unit attuned to the
specific spectrum of human vision. 1 W/m2 of just visible
light is equivalent to 683 lx, but no light source is that efficient;
for sunlight on Earth the ratio is only 93 lx/(W/m2). That
means our ideal clear midday equinox at the equator experiences about
100,000 lx at the surface, but it can drop to 1/4 in the shade. By
sunset even lit areas drop to a few hundred lx, and at twilight to 3 lx.
A clear night with a full moon can have around 0.05-0.3 lx, and a
moonless, overcast sky with no nearby light sources can drop to 0.0001
lx.
Artificial lighting falls surprisingly low on this scale. A typical
office space with only artificial lighting has around 3-500 lx, and in
homes and hallways it can be as low as 50 lx. Streetlights will
typically deliver around 5 lx to the sidewalk.
Given all this, we can state as a rule of thumb that about 1/1,000 the
light delivered to a surface directly facing sunlight on Earth’s surface
(50 lx) could still be considered well-lit, and 1/1,000 of that (0.05
lx) is still marginally visible, at least by human standards. Now,
accounting for the specific lux delivered to a surface by a specific
star through a specific atmosphere is a devilishly complicated task, so
perhaps it’s simpler to just compare the effect of distance from the
star on irradiance, the total light delivered to the top of the
atmosphere without accounting for human perception:
I
= irradiance (relative to Earth)
L
= luminosity of light source (relative to sun)
d
= distance from light source (AU)
For multiple light sources that illuminate the same side of an object,
the irradiances can be independently calculated and added together. If
you have a star with a different spectrum from the sun, multiplying this
value by 10(bolometric correction)/2.5 should roughly reflect
how it would appear to a human or organism with similar color and light
intensity perception.
Based on these estimates, objects as far as Neptune, 30 AU from the
sun, should appear reasonably well-lit, and objects in the Oort cloud as
far as 1000 AU away should remain visible from up close—though in
reality it will depend on their albedo.
But if the luminosity of the light source is not known—e.g. if it is a
planet or moon reflecting light from elsewhere—than illuminance on a
surface directly facing that object can be calculated from its apparent
magnitude:
Ev
= illuminance (lx)
M
= apparent magnitude
This means that a body directly overhead with an apparent magnitude of
-18.3 is sufficient to keep a surface well-lit on its own and -11 is the
threshold for a surface to be marginally visible.

|
| D. Aguilar/Harvard-Smithsonian Center for Astrophysics |
Calling in the Magratheans
Bearing all the possibilities in mind, I’ve put together a system of
plausible planets for Teacup A. Like I said last time, the idea is to
get something that resembles our solar system, but with a few
interesting choices—some of which won’t quite make sense until the next
post. (Note that I first decided on the gas giant radii before finding
planetsynth; based on that program they imply fairly low metallicities
but are still plausible, so I've kept them.)
Teacup Ab
(m = 0.1 Earth masses,
r = 0.46 Earth radii) is a
Mercury analog, though slightly larger and less dense (it’s still the
densest planet in the system). Its orbital period is only 20.46 Earth
days but, being tidal-locked with low obliquity and eccentricity, this
causes little change to its surface. Its dayside is a sweltering
hellscape reaching over 800 K, but its nightside remains below freezing
and, thanks to delivery of ice by comets, has a permanent icecap. Given
that the other planets seem to be acting to continuously pump up its
eccentricity, it probably experiences a good deal of tidal heating and
may experience occasional bursts of volcanism, so much of the dayside is
covered in flood basalts with some impact-generated regolith, and
overall it isn’t as cratered as Mercury. I was tempted to move this
planet further in and make a lava-ice world, but I wasn’t sure how the
clouds of escaping atmosphere might affect sunlight levels further out
in the solar system (plus it would make those simulations with Orbe in
the last post painfully slow). Perhaps I’ll come back to the idea
another time.
Teacup Ac
(m = 0.5,
r = 0.87) is a dry desert
planet with a fairly thin but significant N2 atmosphere.
Though close enough to be tidal-locked, due to its eccentricity of 0.15
it has a 3:2 spin-orbit resonance, meaning a synodic day is twice the
length of the year; 27.29 Earth days. It also has a small obliquity of
5°, which in combination with its resonance means that there are small
regions near each pole that experience direct sunlight only briefly
during their respective summers, and some deep valleys that never
experience direct sunlight at all. What little surface moisture the
planet has will gather in ice caps here, which will spread outwards and
melt near the edges. So even though most of the planet is sweltering
hot—over 400K near the equator in midday—there are small temperate
regions with liquid surface water. Like Mars there are regions of flood
basalt and iron oxide dust, but the planet had a wetter period in its
distant past when it formed some andesite that has since eroded into
lighter-colored silica dust. There are also some salt flats from former
lake basins, though many have been covered by lava flows since
then.
Teacup Ad
(m = 1.6,
r = 1.44) is a super-Earth
waterworld, about a quarter water by mass. The ocean is underlain by
high-pressure ice and covered by a thick H2O/N2
atmosphere. Days are around 130 hours long, which means there are
permanent cloud formations that somewhat cool the planet, though a
strong greenhouse effect keeps the ocean surface close to boiling. The
planet is continuously losing mass to space, and has already lost a
significant portion of its oceans.
Teacup Ad I (m = 0.03,
r = 0.35) is mutually
tidally locked to Ad—the month and days of both bodies are all the same
length. Tidal forces help spur occasional volcanism, and much of the
surface is covered in flood basalts, but the planet is just about too
small to hold onto more than a tenuous CO2 atmosphere. The
planet and moon both do decent jobs of lighting each other during their
respective nights; Ad has a diameter of 5.7° and a magnitude of -19.8 as
seen from Ad I at midnight (with no eclipse), giving it 177 lx of
illumination. Eclipses are also common close to the planet’s equinox,
lasting up to 2 hours at any one spot on Ad I.
Teacup Ae
(m = 0.8,
r = 0.96) is our Earth
analog. Its surface is a mix of water oceans, vegetated continents, and
polar icecaps, and it has a 2 bar N2/O2
atmosphere. It has a somewhat smaller metallic core than Earth—25% the
total mass—which gives it a lower density and surface gravity 86% that
of Earth. Combined with the thicker atmosphere, this should make flight
and eventually space travel easier. Naturally we’ll be spending a lot of time with this planet, so I won’t
say much more now.
Teacup Ae I (m = 1.7*10-10,
r = 0.00084) is a small
captured asteroid, similar in size and composition to Mars’s moon
Deimos.
It’s mostly rock, but contains some internal voids and pockets of ice.
From the surface of Ae it looks similar to a planet or satellite seen
from Earth, but its speed and direction should make it stand out.
Teacup Ae II (m = 0.004,
r = 0.18) is a rough analog
to Earth’s moon, with a surface of dark regolith and flood basalts. It
has a similar apparent diameter and magnitude to our moon, but because
Teacup A appears larger than our sun at this distance, Ae never
experiences a total eclipse.
Teacup Af
(m = 120,
r = 9.64) is a Saturn analog
of 0.38 Jupiter masses. Its mass should put it near the peak size a cool
gas giant can achieve, but I’ve shrunk it down a little to reflect its
temperature and age. Given its equilibrium temperature and some internal
heating, we’ll say it has white bands of water clouds near its
equator transitioning to more Jupiter-like red and orange bands towards
the poles..
Teacup Af I (m = 0.00005, r = 0.041), III (m =
0.0004, r = 0.084), and IV (m = 0.00015, r = 0.061)
are all small rocky moons.
Teacup Af II (m = 0.000009, r = 0.021) is an unusually
dense body, 60% metallic by mass, as the result of some collision long
ago.
Teacup Af V (m = 0.2, r = 0.65) is the largest moon in
the system, with properties broadly similar to Mars with a rusty red
exterior, though it’s colder and has a somewhat thicker CO2
atmosphere.
Teacup Ag
(m = 0.0001,
r = 0.061) is a small Ceres
analog, the largest body in an asteroid belt nestled between the
system’s 2 largest giants. Like Ceres, Ag formed when its orbit was inside the iceline, and today it remains 20%
ice with a thin covering of dust. The interior isn’t fully
differentiated and produces too little heat to cause anything more
dramatic than occasional cryovolcanism.
Teacup Ah
(m = 400,
r = 11.10) is the system’s
most massive and largest planet. It receives similar sunlight to Saturn,
and so has a similar, large beige exterior, though distinct weather
bands can be seen on close inspection.
Teacup Ah I (m = 10-7, r = 0.0078) and II (m
= 10-8, r = 0.0037) are both primarily
irregularly-shaped icy bodies, held together by cohesion more than
gravity.
Teacup Ah III (m = 0.025, r = 0.33) is our Io analogue,
though a big larger. Tidal heating from Ah causes constant, widespread
volcanism, giving it a yellow sulfur exterior.
Teacup Ah IV (m = 0.02, r = 0.33) is our Europa analogue
with a rocky interior, icy surface, and subsurface water ocean and
cryovolcanism thanks to tidal heating.
Teacup Ah V (m = 0.03, r = 0.41) is our Titan analogue,
with a Nitrogen atmosphere and methane oceans on its icy crust.
Teacup Ah VI (m = 0.005, r = 0.25) and VII (m =
0.0008, r = 0.15), the trojan moons, are both small, icy
bodies.
Teacup Ai
(m = 3, r = 1.82) is half water and other volatiles by
mass, and has a substantial subsurface water ocean under an icy surface
tinted pink by methane and colored by regions of cryovolcanism and
cryolava flows. It has a substantial hydrogen atmosphere, and I'll give
it some nitrogen lakes at the equator and seasonally at the poles (I
didn't do this when I first wrote this post but on reanalysis I think
it's appropriate if we assume 10-20 K of greenhouse heating).
Teacup Ai I (m = 0.0001, r = 0.078), II (m =
0.001, r = 0.16), and III (m = 0.0003, r = 0.11)
are all icy moons with no particularly unusual features to report just
now.
Teacup Aj
(m = 25, r = 4.48) is our
Neptune analog, a blue ice giant out at the edge of the system. Though
in reality it’s about twice as far from Teacup A as the point with
equivalent sunlight as Neptune.
Teacup Aj I (m = 0.00002, r = 0.046), II (m =
0.0006, r = 0.14), and IV (m = 0.00005, r = 0.058)
are, again, typical icy moons.
Teacup Aj III (m = 0.01, r = 0.33) is a bit larger and
has a subsurface ocean.
To round things off, Here are the peak apparent diameters and
magnitudes of many of these objects to astronomers on Ae (in visual
light):
|
Body
|
A
|
Ad
|
Ae I
|
Ae II
|
Af
|
Ah
|
Aj
|
B
|
|
Apparent
Diameter
|
0.78
|
0.06
|
0.01
|
0.54
|
0.10
|
0.015
|
0.001
|
0.001
|
|
Apparent
Magnitude
|
-25.9
|
-5.0
|
-3.4
|
-12.4
|
-8.4
|
-0.35
|
8.2
|
-9.4
|
This has a couple interesting implications for astronomers on Ae: At
ideal points, The nearest planets will appear large enough to pick out
some surface features, in particular the climate bands on Af, and their
change in apparent size throughout the year will be obvious. Af and
Teacup B will both shine brighter in Ae’s sky than any star in ours.
Presuming human-like eyes, Ah will be dim but visible, Ag (apparent
magnitude of 5.7) and Ai (apparent magnitude of 6.2) might be just on
the cusp of visibility, and Aj will pass unnoticed before telescopes. Af
V (apparent magnitude of -2.2) should also appear as a distinct object,
as much as 2° apart from Af, so that could help head off any ideas that
objects orbit only around Ae.
As a final little note, since first writing up this post, the creator of
Stellar System Creator
has worked a lot of the math into their program, and you can use it to
make some neat renders of your planets; here's one I worked up of Teacup A
(just using the default planet textures, but you can pretty easily import
a wider variety).
In Summary
- A planet's radius can be estimated from its mass in various ways depending on the type of planet.
- Rough estimates can be made from mass alone based on the like composition for planets of Earth-like, Neptune-like, or Jupiter-like masses.
- For rocky worlds and cool waterworlds, it can be estimated from mass and composition.
- For post-runaway waterworlds, it can be estimated from mass, composition, and temperature.
- For gas giants, it can be estimated from mass, composition, insolation, and age.
- Planetary surface temperature depends on a balance between incoming light and outgoing heat, with factors like surface albedo and planetary greenhouse effects altering the rate of absorption and emission.
- Solid planetary surfaces should usually be rocky or icy, but various other more unusual surfaces are plausible.
- Planetary atmospheres are controlled by balances of sources (outgassing, chemistry) and sinks (sequestration, escape), and may evolve significantly over a planet's history.
- Thermal escape, controlled by a planet's escape velocity and temperature and a gas's molar mass, is one of the the main limitations on atmospheric composition.
- Larger planets should have thicker atmospheres as a general trend but individual planets could greatly vary.
- Surface oceans require appropriate temperature and pressure conditions between a liquid's triple point and critical point.
- Apparent brightness to human eyes falls much more slowly than actual sunlight in the outer solar system.
- Daily lunar eclipses, interplanetary eclipses, and reflected-light days are all possible on other bodies.
Notes
Apparently the dynamics of tidal heating were first described by George
Darwin, Charles’ grandson.
Okay, an explanation of the "centrifugal forces are fake" thing if anyone
needs it: Physicists will sometimes refer to centrifugal forces as
fictitious forces because, if you were observing a rotating
system from afar in an inertial reference frame (meaning you are not
accelerating in any way) and you described that system in your reference
frame, you wouldn't see any centrifugal forces: instead you'd see that
objects tend to move in straight lines, and getting them to rotate in a
circle requires a continuous centripetal force that pulls
inwards towards the axis of rotation (for planets, this is
gravity).
However, you can mathematically describe that system with a rotating
reference frame, where you assume that everything is moving in a circle and
only describe motion relative to that frame; in that case, there are no
centripetal forces, but instead there appear to be centrifugal forces
pulling everything outwards away from the axis of
rotation—because the straight path objects within the rotating system would
follow under inertia alone would take them away from the central axis, the
inward centripetal force is required to keep them rotating in a circle, and
by Newton's 3rd law there must be an equal and opposite outward force in
turn.
There are some issues with this non-inertial reference frame; distant
objects not rotating with the system will appear to behave in impossible
ways and even in the system odd behavior can appear like the Coriolis
effect. But if you're aware of the limits of your framework, account for the
odd behavior where you can, and care only about the behavior of objects
within the rotating system, these aren't huge problems; a description of
centrifugal forces can be used to accurately predict how objects behave on
the rotating surface of a planet, and do so a lot easier than having to
describe everything in an inertial frame and deal with constant changes in
the direction of gravitational and centripetal forces.
Physicist will describe systems with these sorts of convenient if imperfect
reference frames all the time. And, according to general relativity, gravity
is also a fictitious that appears in reference frames that don't
account for the curved geometry of 4-dimensional spacetime. Presuming we
don't want to pull out Einstein's field equations to answer simple questions
about the motion of objects near planets, I think we're fine using
fictitious forces where they produce reasonably accurate predictions.
Buy me a cup of tea (on Patreon)
Part IVc
1 Chen, J., & Kipping, D. (2016). Probabilistic forecasting of the
masses and radii of other worlds. The Astrophysical Journal, 834(1),
17.
2 Fortney, J. J., Marley, M. S., & Barnes, J. W. (2007). Planetary radii across five orders of magnitude in mass and stellar insolation: application to transits. The Astrophysical Journal, 659(2), 1661.
3 Spaargaren, R. J., Wang, H. S., Mojzsis, S. J., Ballmer, M. D., & Tackley, P. J. (2022). Plausible constraints on the range of bulk terrestrial exoplanet compositions in the Solar neighbourhood. arXiv preprint arXiv:2211.01800.
4 Putirka, K. D., & Xu, S. (2021). Polluted white dwarfs reveal exotic mantle rock types on exoplanets in our solar neighborhood. Nature communications, 12(1), 6168.
5 Elkins-Tanton, L. T., & Seager, S. (2008). Coreless terrestrial exoplanets. The Astrophysical Journal, 688(1), 628.
6 Aguichine, A., Mousis, O., Deleuil, M., & Marcq, E. (2021). Mass–Radius Relationships for Irradiated Ocean Planets. The Astrophysical Journal, 914(2), 84.
7 Aguichine Artem, Olivier Mousis, Magali Deleuil, & Emmanuel Marcq. (2021). Mass-radius relationships and fitted coefficients (v1.0.0) [Data set]. Zenodo. https://doi.org/10.5281/zenodo.4552188
8 Müller, S., & Helled, R. (2021). Synthetic evolution tracks of giant planets. Monthly Notices of the Royal Astronomical Society, 507(2), 2094-2102.
9 Lineweaver, C. H., & Norman, M. (2010). The potato radius: a lower minimum size for dwarf planets. arXiv preprint arXiv:1004.1091.
10 "Rotational Flattening" in "Newtonian Dynamics", Richard Fitzpatrick, University of Texas at Austin
11 Kadoya, S., & Tajika, E. (2019). Outer limits of the habitable zones in terms of climate mode and climate evolution of earth-like planets. The Astrophysical Journal, 875(1), 7.
12 Shellnutt, J. G. (2013). Petrological modeling of basaltic rocks from Venus: a case for the presence of silicic rocks. Journal of Geophysical Research: Planets, 118(6), 1350-1364.
13 Bloch, D. (2019). The Leaching of Sub-Florescent Soils as Used in the Ancient Qanat Karez Technology to Produce a Modern Cheap Solution for Controlling and Adjusting Marginal World Albedo. Journal of Earth and Environmental Science Research. SRC/JEESR-103. DOI: https://doi. org/10.47363/JEESR/2019 (1), 103, 3.
14 Unterborn, C. T., Hull, S. D., Stixrude, L. P., Teske, J. K., Johnson, J. A., & Panero, W. R. (2017). Stellar chemical clues as to the rarity of exoplanetary tectonics. arXiv preprint arXiv:1706.10282.
15 Mansfield, M., Kite, E. S., Hu, R., Koll, D. D., Malik, M., Bean, J. L., & Kempton, E. M. R. (2019). Identifying atmospheres on rocky exoplanets through inferred high albedo. The Astrophysical Journal, 886(2), 141.
16 Dorn, C., Harrison, J. H., Bonsor, A., & Hands, T. O. (2019). A new class of Super-Earths formed from high-temperature condensates: HD219134 b, 55 Cnc e, WASP-47 e. Monthly Notices of the Royal Astronomical Society, 484(1), 712-727.
17 Wurm, G., Trieloff, M., & Rauer, H. (2013). Photophoretic separation of metals and silicates: The formation of Mercury-like planets and metal depletion in chondrites. The Astrophysical Journal, 769(1), 78.
18 Price, E. M., & Rogers, L. A. (2020). Tidally distorted, iron-enhanced exoplanets closely orbiting their stars. The Astrophysical Journal, 894(1), 8.
19 Beech, M., & Peltier, L. (2017). The vulcanoid asteroids: Past, present and future. American Journal of Astronomy and Astrophysics, 5(3), 28.
20 Benz, W., Anic, A., Horner, J., & Whitby, J. A. (2008). The origin of Mercury. Mercury, 7-20.
21 Reinhardt, C., Meier, T., Stadel, J. G., Otegi, J. F., & Helled, R. (2022). Forming iron-rich planets with giant impacts. Monthly Notices of the Royal Astronomical Society, 517(3), 3132-3143.
22 Johnson, B. C., Sori, M. M., & Evans, A. J. (2020). Ferrovolcanism on metal worlds and the origin of pallasites. Nature Astronomy, 4(1), 41-44.
23 Jia, S., & Spruit, H. C. (2017). Instability of mass transfer in a planet–star system. Monthly Notices of the Royal Astronomical Society, 465(1), 149-160.
24 Schaefer, L., & Fegley Jr, B. (2004). Heavy metal frost on Venus. Icarus, 168(1), 215-219.
25 Turtle, E. P., Perry, J. E., Hayes, A. G., Lorenz, R. D., Barnes, J. W., McEwen, A. S., ... & Stofan, E. R. (2011). Rapid and extensive surface changes near Titan’s equator: Evidence of April showers. science, 331(6023), 1414-1417.
26 Seager, S., Kuchner, M., Hier-Majumder, C. A., & Militzer, B. (2007). Mass-radius relationships for solid exoplanets. The Astrophysical Journal, 669(2), 1279.
27 Henin, B. (2018). The Frost Line. In Exploring the Ocean Worlds of Our Solar System (pp. 21-31). Springer, Cham.
28 Qi, C., Öberg, K. I., Wilner, D. J., d’Alessio, P., Bergin, E., Andrews, S. M., ... & Van Dishoeck, E. F. (2013). Imaging of the CO snow line in a solar nebula analog. Science, 341(6146), 630-632.
29 Lehmer, O. R., Catling, D. C., & Zahnle, K. J. (2017). The longevity of water ice on Ganymedes and Europas around migrated giant planets. The Astrophysical Journal, 839(1), 32.
30 Johnson, B. C., Sheppard, R. Y., Pascuzzo, A. C., Fisher, E. A., & Wiggins, S. E. (2017). Porosity and salt content determine if subduction can occur in Europa's ice shell. Journal of Geophysical Research: Planets, 122(12), 2765-2778.
31 Soderblom, L. A., Kieffer, S. W., Becker, T. L., Brown, R. H., Cook, A. F., Hansen, C. J., ... & Shoemaker, E. M. (1990). Triton's geyser-like plumes: Discovery and basic characterization. Science, 250(4979), 410-415.
32 William B. McKinnon, Randolph L. Kirk, Chapter 40 - Triton, Editor(s): Tilman Spohn, Doris Breuer, Torrence V. Johnson, Encyclopedia of the Solar System (Third Edition), Elsevier, 2014, Pages 861-881, ISBN 9780124158450, https://doi.org/10.1016/B978-0-12-415845-0.00040-2.
33 Singer, K. N., White, O. L., Schmitt, B., Rader, E. L., Protopapa, S., Grundy, W. M., ... & Ennico-Smith, K. (2022). Large-scale cryovolcanic resurfacing on Pluto. Nature communications, 13(1), 1542.
34 Coelho, L. F., Madden, J., Kaltenegger, L., Zinder, S., Philpot, W., Esquível, M. G., ... & Martins, Z. (2022). Color catalogue of life in ice: surface biosignatures on icy worlds. Astrobiology, 22(3), 313-321.
35 Brewer, J. M., & Fischer, D. A. (2016). C/O and Mg/Si ratios of stars in the solar neighborhood. The Astrophysical Journal, 831(1), 20.
36 Lichtenberg, T., & Krijt, S. (2021). System-level fractionation of carbon from disk and planetesimal processing. The Astrophysical Journal Letters, 913(2), L20.
37 Wilson, H. F., & Militzer, B. (2014). INTERIOR PHASE TRANSFORMATIONS AND MASS–RADIUS RELATIONSHIPS OF SILICON–CARBON PLANETS. The Astrophysical Journal, 793(1), 34.
38 Unterborn, C. T., Kabbes, J. E., Pigott, J. S., Reaman, D. M., & Panero, W. R. (2014). The role of carbon in extrasolar planetary geodynamics and habitability. The Astrophysical Journal, 793(2), 124.
39 Allen-Sutter, H., Garhart, E., Leinenweber, K., Prakapenka, V., Greenberg, E., & Shim, S. H. (2020). Oxidation of the interiors of carbide exoplanets. The Planetary Science Journal, 1(2), 39.
40 Hakim, K., Spaargaren, R., Grewal, D. S., Rohrbach, A., Berndt, J., Dominik, C., & Van Westrenen, W. (2019). Mineralogy, structure, and habitability of carbon-enriched rocky exoplanets: a laboratory approach. Astrobiology, 19(7), 867-884.
41 Dangi, B. B., Kim, Y. S., Krasnokutski, S. A., Kaiser, R. I., & Bauschlicher Jr, C. W. (2015). Toward the Formation of Carbonaceous Refractory Matter in High Temperature Hydrocarbon-Rich Atmospheres of Exoplanets Upon Micrometeoroid Impact. The Astrophysical Journal, 805(1), 76.
42 Futó, P. P. POSSIBLE FORMATION SCENARIOS AND MINERALOGICAL TYPES OF CARBON-RICH SOLID.
43 Maynard-Casely, H. E., Cable, M. L., Malaska, M. J., Vu, T. H., Choukroun, M., & Hodyss, R. (2018). Prospects for mineralogy on Titan. American Mineralogist: Journal of Earth and Planetary Materials, 103(3), 343-349.
44 Lopes, R. M., & Williams, D. A. (2015). Volcanism on Io. In The encyclopedia of volcanoes (pp. 747-762). Academic Press.
45 Essack, Z., Seager, S., & Pajusalu, M. (2020). Low-albedo surfaces of lava worlds. The Astrophysical Journal, 898(2), 160.
46 O’Malley-James, J. T., & Kaltenegger, L. (2018). Biofluorescent worlds: Global biological fluorescence as a biosignature. Monthly Notices of the Royal Astronomical Society, 481(2), 2487-2496.
47 Huang, Y. F., & Yu, Y. B. (2017). Searching for strange quark matter objects in exoplanets. The Astrophysical Journal, 848(2), 115.
48 Zapata, J., & Negreiros, R. (2020). Orbital Properties and Gravitational-wave Signatures of Strangelet Crystal Planets. The Astrophysical Journal, 892(1), 67.
49 Kuerban, A., Geng, J. J., Huang, Y. F., Zong, H. S., & Gong, H. (2020). Close-in Exoplanets as Candidates for Strange Quark Matter Objects. The Astrophysical Journal, 890(1), 41.
50 Alford, M. G., Han, S., & Reddy, S. (2012). Strangelet dwarfs. Journal of Physics G: Nuclear and Particle Physics, 39(6), 065201.
51 Lammer, H., Stökl, A., Erkaev, N. V., Dorfi, E. A., Odert, P., Güdel, M., ... & Leitzinger, M. (2014). Origin and loss of nebula-captured hydrogen envelopes from ‘sub’-to ‘super-Earths’ in the habitable zone of Sun-like stars. Monthly Notices of the Royal Astronomical Society, 439(4), 3225-3238.
52 Wang, Z., Zhou, Y., & Liu, Y. (2022). The escape mechanisms of the proto-atmosphere on terrestrial planets:“boil-off” escape, hydrodynamic escape and impact erosion. Acta Geochimica, 41(4), 592-606.
53 Williams, D. M., Kasting, J. F., & Wade, R. A. (1997). Habitable moons around extrasolar giant planets. Nature, 385(6613), 234-236.
54 Zahnle, K. J., Lupu, R., Catling, D. C., & Wogan, N. (2020). Creation and evolution of impact-generated reduced atmospheres of early Earth. The Planetary Science Journal, 1(1), 11.
55 Gunell, H., Maggiolo, R., Nilsson, H., Wieser, G. S., Slapak, R., Lindkvist, J., ... & De Keyser, J. (2018). Why an intrinsic magnetic field does not protect a planet against atmospheric escape. Astronomy & Astrophysics, 614, L3.
56 Swain, M. R., Estrela, R., Roudier, G. M., Sotin, C., Rimmer, P. B., Valio, A., ... & Zellem, R. T. (2021). Detection of an atmosphere on a rocky exoplanet. The Astronomical Journal, 161(5), 213.
57 Wordsworth, R., & Pierrehumbert, R. (2013). Hydrogen-nitrogen greenhouse warming in Earth's early atmosphere. science, 339(6115), 64-67.
58 Visions 2200, "Extrasolar Speculations"; further details unclear but it appears to be stably archived.
59 Sudarsky, D., Burrows, A., & Pinto, P. (2000). Albedo and reflection spectra of extrasolar giant planets. The Astrophysical Journal, 538(2), 885.
60 Gao, P., Marley, M. S., Zahnle, K., Robinson, T. D., & Lewis, N. K. (2017). Sulfur hazes in giant exoplanet atmospheres: impacts on reflected light spectra. The Astronomical Journal, 153(3), 139.
61 Parmentier, V., Fortney, J. J., Showman, A. P., Morley, C., & Marley, M. S. (2016). Transitions in the cloud composition of hot Jupiters. The Astrophysical Journal, 828(1), 22.
62 "Neptunian and Jovian Cloud Types for Orion's Arm", google doc; if this disappears, check out the EWoCS page on Orion's Arm based on it https://orionsarm.com/eg-article/5e724eb65b934
63 Helled, R., Movshovitz, N., & Nettelmann, N. (2022). The nature of gas giant planets. arXiv preprint arXiv:2202.10046.
64 Hu, R., Seager, S., & Yung, Y. L. (2015). Helium atmospheres on warm Neptune-and sub-Neptune-sized exoplanets and applications to GJ 436b. The Astrophysical Journal, 807(1), 8.
65 Bailes, M., Bates, S. D., Bhalerao, V., Bhat, N., Burgay, M., Burke-Spolaor, S., ... & van Straten, W. (2011). Transformation of a star into a planet in a millisecond pulsar binary. Science, 333(6050), 1717-1720.
66 Hu, R., & Seager, S. (2014). Photochemistry in terrestrial exoplanet atmospheres. III. Photochemistry and thermochemistry in thick atmospheres on super Earths and mini Neptunes. The Astrophysical Journal, 784(1), 63.
67 Hu, R., & Thomas, T. B. (2022). A nitrogen-rich atmosphere on ancient Mars consistent with isotopic evolution models. Nature Geoscience, 15(2), 106-111.
68 Mikhail, S., & Sverjensky, D. A. (2014). Nitrogen speciation in upper mantle fluids and the origin of Earth's nitrogen-rich atmosphere. Nature Geoscience, 7(11), 816-819.
69 Catling, D. C., & Zahnle, K. J. (2020). The archean atmosphere. Science advances, 6(9), eaax1420.
70 Wordsworth, R. D. (2016). Atmospheric nitrogen evolution on Earth and Venus. Earth and Planetary Science Letters, 447, 103-111.
71 Laneuville, M., Kameya, M., & Cleaves, H. J. (2018). Earth without life: A systems model of a global abiotic nitrogen cycle. Astrobiology, 18(7), 897-914.
72 Krissansen-Totton, J., Schwieterman, E. W., Charnay, B., Arney, G., Robinson, T. D., Meadows, V., & Catling, D. C. (2016). Is the Pale Blue Dot unique? Optimized photometric bands for identifying Earth-like exoplanets. The Astrophysical Journal, 817(1), 31.
73 Meadows, V. S., Reinhard, C. T., Arney, G. N., Parenteau, M. N., Schwieterman, E. W., Domagal-Goldman, S. D., ... & Grenfell, J. L. (2018). Exoplanet biosignatures: understanding oxygen as a biosignature in the context of its environment. Astrobiology, 18(6), 630-662.
74 Watanabe, Y., & Tajika, E. (2021). Atmospheric oxygenation of the early earth and earth-like planets driven by competition between land and seafloor weathering. Earth, Planets and Space, 73(1), 1-10.
75 Krissansen‐Totton, J., Fortney, J. J., Nimmo, F., & Wogan, N. (2021). Oxygen False Positives on Habitable Zone Planets Around Sun‐Like Stars. AGU Advances, 2(2), e2020AV000294.
76 Wordsworth, R., & Pierrehumbert, R. (2014). Abiotic oxygen-dominated atmospheres on terrestrial habitable zone planets. The Astrophysical Journal Letters, 785(2), L20.
77 kumar Kopparapu, R., Wolf, E. T., Arney, G., Batalha, N. E., Haqq-Misra, J., Grimm, S. L., & Heng, K. (2017). Habitable moist atmospheres on terrestrial planets near the inner edge of the habitable zone around M dwarfs. The Astrophysical Journal, 845(1), 5.
78 do Amaral, L. N., Barnes, R., Segura, A., & Luger, R. (2022). The Contribution of M-dwarf Flares to the Thermal Escape of Potentially Habitable Planet Atmospheres. The Astrophysical Journal, 928(1), 12.
79 Hu, R., Peterson, L., & Wolf, E. T. (2020). O2-and CO-rich atmospheres for potentially habitable environments on TRAPPIST-1 planets. The Astrophysical Journal, 888(2), 122.
80 Narita, N., Enomoto, T., Masaoka, S., & Kusakabe, N. (2015). Titania may produce abiotic oxygen atmospheres on habitable exoplanets. Scientific reports, 5(1), 13977.
81 Belcher, C. M., Yearsley, J. M., Hadden, R. M., McElwain, J. C., & Rein, G. (2010). Baseline intrinsic flammability of Earth’s ecosystems estimated from paleoatmospheric oxygen over the past 350 million years. Proceedings of the National Academy of Sciences, 107(52), 22448-22453.
82 Vidaurri, M. R., Bastelberger, S. T., Wolf, E. T., Domagal-Goldman, S., & Kopparapu, R. K. (2022). The Outer Edge of the Venus Zone around Main-sequence Stars. The Planetary Science Journal, 3(6), 137.
83 He, C., Hörst, S. M., Lewis, N. K., Yu, X., Moses, J. I., McGuiggan, P., ... & Vuitton, V. (2020). Sulfur-driven haze formation in warm CO2-rich exoplanet atmospheres. Nature Astronomy, 4(10), 986-993.
84 Schaefer, L., & Fegley, B. (2011). Atmospheric chemistry of Venus-like exoplanets. The Astrophysical Journal, 729(1), 6.
85 Grenfell, J. L., Leconte, J., Forget, F., Godolt, M., Carrión-González, Ó., Noack, L., ... & Turbet, M. (2020). Possible Atmospheric Diversity of Low Mass Exoplanets–Some Central Aspects. Space Science Reviews, 216, 1-38.
86 Zhan, Z., Huang, J., Seager, S., Petkowski, J. J., & Ranjan, S. (2022). Organic Carbonyls Are Poor Biosignature Gases in Exoplanet Atmospheres but May Generate Significant CO. The Astrophysical Journal, 930(2), 133.
87 Needham, D. H., & Kring, D. A. (2017). Lunar volcanism produced a transient atmosphere around the ancient Moon. Earth and Planetary Science Letters, 478, 175-178.
88 Schwieterman, E. W., Olson, S. L., Pidhorodetska, D., Reinhard, C. T., Ganti, A., Fauchez, T. J., ... & Lyons, T. W. (2022). Evaluating the Plausible Range of N2O Biosignatures on Exo-Earths: An Integrated Biogeochemical, Photochemical, and Spectral Modeling Approach. The Astrophysical Journal, 937(2), 109.
89 Airapetian, V. S., Barnes, R., Cohen, O., Collinson, G. A., Danchi, W. C., Dong, C. F., ... & Yamashiki, Y. (2020). Impact of space weather on climate and habitability of terrestrial-type exoplanets. International Journal of Astrobiology, 19(2), 136-194.
90 Lammer, H., Schiefer, S. C., Juvan, I., Odert, P., Erkaev, N. V., Weber, C., ... & Hanslmeier, A. (2014). Origin and stability of exomoon atmospheres: implications for habitability. Origins of life and Evolution of Biospheres, 44, 239-260.
91 Pierrehumbert, R. T., & Ding, F. (2016). Dynamics of atmospheres with a non-dilute condensible component. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 472(2190), 20160107.
92 Kuchner, M. J. (2003). Volatile-rich Earth-mass planets in the habitable zone. The Astrophysical Journal, 596(1), L105.
93 Kaltenegger, L., Sasselov, D., & Rugheimer, S. (2013). Water-planets in the habitable zone: atmospheric chemistry, observable features, and the case of Kepler-62e and-62f. The Astrophysical Journal Letters, 775(2), L47.
94 Goldblatt, C. (2015). Habitability of waterworlds: runaway greenhouses, atmospheric expansion, and multiple climate states of pure water atmospheres. Astrobiology, 15(5), 362-370.
95 Arnscheidt, C. W., Wordsworth, R. D., & Ding, F. (2019). Atmospheric evolution on low-gravity waterworlds. The Astrophysical Journal, 881(1), 60.
96 Del Genio, A. D., Kiang, N. Y., Way, M. J., Amundsen, D. S., Sohl, L. E., Fujii, Y., ... & Kelley, M. (2019). Albedos, equilibrium temperatures, and surface temperatures of habitable planets. The Astrophysical Journal, 884(1), 75.
97 Pluriel, W., Marcq, E., & Turbet, M. (2019). Modeling the albedo of Earth-like magma ocean planets with H2O-CO2 atmospheres. Icarus, 317, 583-590.
98 Liggins, P., Jordan, S., Rimmer, P. B., & Shorttle, O. (2022). Growth and evolution of secondary volcanic atmospheres: I. Identifying the geological character of hot rocky planets. Journal of Geophysical Research: Planets, 127(7), e2021JE007123.
99 Civiš, S., Knížek, A., Ivanek, O., Kubelík, P., Zukalová, M., Kavan, L., & Ferus, M. (2017). The origin of methane and biomolecules from a CO2 cycle on terrestrial planets. Nature Astronomy, 1(10), 721-726.
100 Haqq-Misra, J. D., Domagal-Goldman, S. D., Kasting, P. J., & Kasting, J. F. (2008). A revised, hazy methane greenhouse for the Archean Earth. Astrobiology, 8(6), 1127-1137.
101 Crow, C. A., McFadden, L. A., Robinson, T., Meadows, V. S., Livengood, T. A., Hewagama, T., ... & Wellnitz, D. (2011). Views from EPOXI: colors in our solar system as an analog for extrasolar planets. The Astrophysical Journal, 729(2), 130.
102 Hu, R., Seager, S., & Bains, W. (2013). Photochemistry in terrestrial exoplanet atmospheres. II. H2S and SO2 photochemistry in anoxic atmospheres. The Astrophysical Journal, 769(1), 6.
103 Loftus, K., Wordsworth, R. D., & Morley, C. V. (2019). Sulfate aerosol hazes and SO2 gas as constraints on rocky exoplanets’ surface liquid water. The Astrophysical Journal, 887(2), 231.
104 Zilinskas, M., Miguel, Y., Mollière, P., & Tsai, S. M. (2020). Atmospheric compositions and observability of nitrogen-dominated ultra-short-period super-Earths. Monthly Notices of the Royal Astronomical Society, 494(1), 1490-1506.
105 Fegley, B., Jacobson, N. S., Williams, K. B., Plane, J. M. C., Schaefer, L., & Lodders, K. (2016). Solubility of rock in steam atmospheres of planets. The Astrophysical Journal, 824(2), 103.
106 Schaefer, L., & Fegley, B. (2009). Chemistry of silicate atmospheres of evaporating super-Earths. The Astrophysical Journal, 703(2), L113.
107 Lammer, H., Scherf, M., Ito, Y., Mura, A., Vorburger, A., Guenther, E., ... & Odert, P. (2022). The exosphere as a boundary: Origin and evolution of airless bodies in the inner solar system and beyond including planets with silicate atmospheres. Space science reviews, 218(3), 15.
108 Ranjan, S., Seager, S., Zhan, Z., Koll, D. D., Bains, W., Petkowski, J. J., ... & Lin, Z. (2022). Photochemical Runaway in Exoplanet Atmospheres: Implications for Biosignatures. The Astrophysical Journal, 930(2), 131.
109 Rimmer, P. B., & Rugheimer, S. (2019). Hydrogen cyanide in nitrogen-rich atmospheres of rocky exoplanets. Icarus, 329, 124-131.
110 Luque, R., & Pallé, E. (2022). Density, not radius, separates rocky and water-rich small planets orbiting M dwarf stars. Science, 377(6611), 1211-1214.
111 Madhusudhan, N., Piette, A. A., & Constantinou, S. (2021). Habitability and biosignatures of Hycean worlds. The Astrophysical Journal, 918(1), 1.
112 Cowan, N. B., & Abbot, D. S. (2014). Water cycling between ocean and mantle: super-Earths need not be waterworlds. The Astrophysical Journal, 781(1), 27.
113 Schaefer, L., & Sasselov, D. (2015). The persistence of oceans on Earth-like planets: Insights from the deep-water cycle. The Astrophysical Journal, 801(1), 40.
114 Noack, L., Höning, D., Rivoldini, A., Heistracher, C., Zimov, N., Journaux, B., ... & Bredehöft, J. H. (2016). Water-rich planets: How habitable is a water layer deeper than on Earth?. Icarus, 277, 215-236.
115 Nixon, M. C., & Madhusudhan, N. (2021). How deep is the ocean? Exploring the phase structure of water-rich sub-Neptunes. Monthly Notices of the Royal Astronomical Society, 505(3), 3414-3432.
116 Renno, N. O., Fischer, E., Martínez, G., & Hanley, J. (2021). Complex Brines and Their Implications for Habitability. Life, 11(8), 847.
117 Halevy, I., Alesker, M., Schuster, E. M., Popovitz-Biro, R., & Feldman, Y. (2017). A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nature Geoscience, 10(2), 135-139.
118 Gallardo, V. A., & Espinoza, C. (2008, August). The evolution of ocean color. In Instruments, Methods, and Missions for Astrobiology XI (Vol. 7097, pp. 128-134). SPIE.
119 Gueneli, N., McKenna, A. M., Ohkouchi, N., Boreham, C. J., Beghin, J., Javaux, E. J., & Brocks, J. J. (2018). 1.1-billion-year-old porphyrins establish a marine ecosystem dominated by bacterial primary producers. Proceedings of the National Academy of Sciences, 115(30), E6978-E6986.
120 Cordier, D., Mousis, O., Lunine, J. I., Lebonnois, S., Rannou, P., Lavvas, P., ... & Ferreira, A. G. M. (2012). Titan's lakes chemical composition: sources of uncertainties and variability. Planetary and Space Science, 61(1), 99-107.
121 Hayes, A. G., Lorenz, R. D., & Lunine, J. I. (2018). A post-Cassini view of Titan’s methane-based hydrologic cycle. Nature Geoscience, 11(5), 306-313.
122 Faulk, S. P., Lora, J. M., Mitchell, J. L., & Milly, P. C. D. (2020). Titan’s climate patterns and surface methane distribution due to the coupling of land hydrology and atmosphere. Nature Astronomy, 4(4), 390-398.
123 Cordier, D., & Carrasco, N. (2019). The floatability of aerosols and wave damping on Titan’s seas. Nature Geoscience, 12(5), 315-320.
124 GLIESE, S. S. M. O., & d Steven, M. AN INVESTIGATION OF EXTENSIVE TIDALLY HEATED SUPER-EARTHS (SUPER-IOS) USING A. Icarus, 226, 1743-1761.
125 Woitke, P., Herbort, O., Helling, C., Stüeken, E., Dominik, M., Barth, P., & Samra, D. (2021). Coexistence of CH4, CO2, and H2O in exoplanet atmospheres. Astronomy & Astrophysics, 646, A43.
126 Hogenboom, D. L., Kargel, J. S., Holden, T. C., & Ganasan, J. (1994, March). The ammonia-water phase diagram and phase volumes to 4 kbars. In Lunar and Planetary Science Conference (Vol. 25, p. 555).
127 Graham, R. J., Lichtenberg, T., & Pierrehumbert, R. T. (2022). CO2 ocean bistability on terrestrial exoplanets. Journal of Geophysical Research: Planets, 127(10), e2022JE007456.
128 Tang, Y., Chen, Q., & Huang, Y. (2006). Early Mars may have had a methanol ocean. Icarus, 180(1), 88-92.
129 Schulze-Makuch, D., Irwin, L. N., Schulze-Makuch, D., & Irwin, L. N. (2018). Life and the Need for a Solvent. Life in the universe: Expectations and constraints, 123-147.
130 Dougherty, A. J., Bartholet, Z. T., Chumsky, R. J., Delano, K. C., Huang, X., & Morris, D. K. (2018). The Liquidus Temperature for Methanol‐Water Mixtures at High Pressure and Low Temperature, With Application to Titan. Journal of Geophysical Research: Planets, 123(12), 3080-3087.
131 Saladino, R., Crestini, C., Pino, S., Costanzo, G., & Di Mauro, E. (2012). Formamide and the origin of life. Physics of life reviews, 9(1), 84-104.
132 Miyazaki, Y., & Korenaga, J. (2022). Inefficient water degassing inhibits ocean formation on rocky planets: An insight from self-consistent mantle degassing models. Astrobiology, 22(6), 713-734.
133 Lupu, R. E., Zahnle, K., Marley, M. S., Schaefer, L., Fegley, B., Morley, C., ... & Fortney, J. J. (2014). The atmospheres of earthlike planets after giant impact events. The Astrophysical Journal, 784(1), 27.
134 Nikolaou, A., Katyal, N., Tosi, N., Godolt, M., Grenfell, J. L., & Rauer, H. (2019). What factors affect the duration and outgassing of the terrestrial magma ocean?. The Astrophysical Journal, 875(1), 11.
135 Hamano, K., Kawahara, H., Abe, Y., Onishi, M., & Hashimoto, G. L. (2015). Lifetime and spectral evolution of a magma ocean with a steam atmosphere: its detectability by future direct imaging. The Astrophysical Journal, 806(2), 216.
136 Rappaport, S., Levine, A., Chiang, E., El Mellah, I., Jenkins, J., Kalomeni, B., ... & Tran, K. (2012). Possible disintegrating short-period super-Mercury orbiting KIC 12557548. The Astrophysical Journal, 752(1), 1.
137 Léger, A., Grasset, O., Fegley, B., Codron, F., Albarede, A. F., Barge, P., ... & Sotin, C. (2011). The extreme physical properties of the CoRoT-7b super-Earth. Icarus, 213(1), 1-11.
138 Boukaré, C. É., Cowan, N. B., & Badro, J. (2022). Deep two-phase, hemispherical magma oceans on lava planets. The Astrophysical Journal, 936(2), 148.
139 Kite, E. S., Fegley Jr, B., Schaefer, L., & Gaidos, E. (2016). Atmosphere-interior exchange on hot, rocky exoplanets. The Astrophysical Journal, 828(2), 80.
140 Xu, Y., Xiao, X., Sun, S., & Ouyang, Z. (1996, March). IR spectroscopic evidence of metal carbonyl clusters in the Jiange H5 chondrite. In Lunar and Planetary Science Conference (Vol. 27).
141 Schulze-Makuch, D. (2013). Extremophiles on alien worlds: what types of organismic adaptations are feasible on other planetary bodies. Habitability of Other Planets and Satellites, 253-265.
142 Bains, W. (2004). Many chemistries could be used to build living systems. Astrobiology, 4(2), 137-167.
143 Flower, P. J. (1996). Transformations from theoretical Hertzsprung-Russell diagrams to color-magnitude diagrams: effective temperatures, BV colors, and bolometric corrections. Astrophysical Journal v. 469, p. 355, 469, 355.
144 "Locating Geosynchronous Satellites", Australian Space Academy
2 Fortney, J. J., Marley, M. S., & Barnes, J. W. (2007). Planetary radii across five orders of magnitude in mass and stellar insolation: application to transits. The Astrophysical Journal, 659(2), 1661.
3 Spaargaren, R. J., Wang, H. S., Mojzsis, S. J., Ballmer, M. D., & Tackley, P. J. (2022). Plausible constraints on the range of bulk terrestrial exoplanet compositions in the Solar neighbourhood. arXiv preprint arXiv:2211.01800.
4 Putirka, K. D., & Xu, S. (2021). Polluted white dwarfs reveal exotic mantle rock types on exoplanets in our solar neighborhood. Nature communications, 12(1), 6168.
5 Elkins-Tanton, L. T., & Seager, S. (2008). Coreless terrestrial exoplanets. The Astrophysical Journal, 688(1), 628.
6 Aguichine, A., Mousis, O., Deleuil, M., & Marcq, E. (2021). Mass–Radius Relationships for Irradiated Ocean Planets. The Astrophysical Journal, 914(2), 84.
7 Aguichine Artem, Olivier Mousis, Magali Deleuil, & Emmanuel Marcq. (2021). Mass-radius relationships and fitted coefficients (v1.0.0) [Data set]. Zenodo. https://doi.org/10.5281/zenodo.4552188
8 Müller, S., & Helled, R. (2021). Synthetic evolution tracks of giant planets. Monthly Notices of the Royal Astronomical Society, 507(2), 2094-2102.
9 Lineweaver, C. H., & Norman, M. (2010). The potato radius: a lower minimum size for dwarf planets. arXiv preprint arXiv:1004.1091.
10 "Rotational Flattening" in "Newtonian Dynamics", Richard Fitzpatrick, University of Texas at Austin
11 Kadoya, S., & Tajika, E. (2019). Outer limits of the habitable zones in terms of climate mode and climate evolution of earth-like planets. The Astrophysical Journal, 875(1), 7.
12 Shellnutt, J. G. (2013). Petrological modeling of basaltic rocks from Venus: a case for the presence of silicic rocks. Journal of Geophysical Research: Planets, 118(6), 1350-1364.
13 Bloch, D. (2019). The Leaching of Sub-Florescent Soils as Used in the Ancient Qanat Karez Technology to Produce a Modern Cheap Solution for Controlling and Adjusting Marginal World Albedo. Journal of Earth and Environmental Science Research. SRC/JEESR-103. DOI: https://doi. org/10.47363/JEESR/2019 (1), 103, 3.
14 Unterborn, C. T., Hull, S. D., Stixrude, L. P., Teske, J. K., Johnson, J. A., & Panero, W. R. (2017). Stellar chemical clues as to the rarity of exoplanetary tectonics. arXiv preprint arXiv:1706.10282.
15 Mansfield, M., Kite, E. S., Hu, R., Koll, D. D., Malik, M., Bean, J. L., & Kempton, E. M. R. (2019). Identifying atmospheres on rocky exoplanets through inferred high albedo. The Astrophysical Journal, 886(2), 141.
16 Dorn, C., Harrison, J. H., Bonsor, A., & Hands, T. O. (2019). A new class of Super-Earths formed from high-temperature condensates: HD219134 b, 55 Cnc e, WASP-47 e. Monthly Notices of the Royal Astronomical Society, 484(1), 712-727.
17 Wurm, G., Trieloff, M., & Rauer, H. (2013). Photophoretic separation of metals and silicates: The formation of Mercury-like planets and metal depletion in chondrites. The Astrophysical Journal, 769(1), 78.
18 Price, E. M., & Rogers, L. A. (2020). Tidally distorted, iron-enhanced exoplanets closely orbiting their stars. The Astrophysical Journal, 894(1), 8.
19 Beech, M., & Peltier, L. (2017). The vulcanoid asteroids: Past, present and future. American Journal of Astronomy and Astrophysics, 5(3), 28.
20 Benz, W., Anic, A., Horner, J., & Whitby, J. A. (2008). The origin of Mercury. Mercury, 7-20.
21 Reinhardt, C., Meier, T., Stadel, J. G., Otegi, J. F., & Helled, R. (2022). Forming iron-rich planets with giant impacts. Monthly Notices of the Royal Astronomical Society, 517(3), 3132-3143.
22 Johnson, B. C., Sori, M. M., & Evans, A. J. (2020). Ferrovolcanism on metal worlds and the origin of pallasites. Nature Astronomy, 4(1), 41-44.
23 Jia, S., & Spruit, H. C. (2017). Instability of mass transfer in a planet–star system. Monthly Notices of the Royal Astronomical Society, 465(1), 149-160.
24 Schaefer, L., & Fegley Jr, B. (2004). Heavy metal frost on Venus. Icarus, 168(1), 215-219.
25 Turtle, E. P., Perry, J. E., Hayes, A. G., Lorenz, R. D., Barnes, J. W., McEwen, A. S., ... & Stofan, E. R. (2011). Rapid and extensive surface changes near Titan’s equator: Evidence of April showers. science, 331(6023), 1414-1417.
26 Seager, S., Kuchner, M., Hier-Majumder, C. A., & Militzer, B. (2007). Mass-radius relationships for solid exoplanets. The Astrophysical Journal, 669(2), 1279.
27 Henin, B. (2018). The Frost Line. In Exploring the Ocean Worlds of Our Solar System (pp. 21-31). Springer, Cham.
28 Qi, C., Öberg, K. I., Wilner, D. J., d’Alessio, P., Bergin, E., Andrews, S. M., ... & Van Dishoeck, E. F. (2013). Imaging of the CO snow line in a solar nebula analog. Science, 341(6146), 630-632.
29 Lehmer, O. R., Catling, D. C., & Zahnle, K. J. (2017). The longevity of water ice on Ganymedes and Europas around migrated giant planets. The Astrophysical Journal, 839(1), 32.
30 Johnson, B. C., Sheppard, R. Y., Pascuzzo, A. C., Fisher, E. A., & Wiggins, S. E. (2017). Porosity and salt content determine if subduction can occur in Europa's ice shell. Journal of Geophysical Research: Planets, 122(12), 2765-2778.
31 Soderblom, L. A., Kieffer, S. W., Becker, T. L., Brown, R. H., Cook, A. F., Hansen, C. J., ... & Shoemaker, E. M. (1990). Triton's geyser-like plumes: Discovery and basic characterization. Science, 250(4979), 410-415.
32 William B. McKinnon, Randolph L. Kirk, Chapter 40 - Triton, Editor(s): Tilman Spohn, Doris Breuer, Torrence V. Johnson, Encyclopedia of the Solar System (Third Edition), Elsevier, 2014, Pages 861-881, ISBN 9780124158450, https://doi.org/10.1016/B978-0-12-415845-0.00040-2.
33 Singer, K. N., White, O. L., Schmitt, B., Rader, E. L., Protopapa, S., Grundy, W. M., ... & Ennico-Smith, K. (2022). Large-scale cryovolcanic resurfacing on Pluto. Nature communications, 13(1), 1542.
34 Coelho, L. F., Madden, J., Kaltenegger, L., Zinder, S., Philpot, W., Esquível, M. G., ... & Martins, Z. (2022). Color catalogue of life in ice: surface biosignatures on icy worlds. Astrobiology, 22(3), 313-321.
35 Brewer, J. M., & Fischer, D. A. (2016). C/O and Mg/Si ratios of stars in the solar neighborhood. The Astrophysical Journal, 831(1), 20.
36 Lichtenberg, T., & Krijt, S. (2021). System-level fractionation of carbon from disk and planetesimal processing. The Astrophysical Journal Letters, 913(2), L20.
37 Wilson, H. F., & Militzer, B. (2014). INTERIOR PHASE TRANSFORMATIONS AND MASS–RADIUS RELATIONSHIPS OF SILICON–CARBON PLANETS. The Astrophysical Journal, 793(1), 34.
38 Unterborn, C. T., Kabbes, J. E., Pigott, J. S., Reaman, D. M., & Panero, W. R. (2014). The role of carbon in extrasolar planetary geodynamics and habitability. The Astrophysical Journal, 793(2), 124.
39 Allen-Sutter, H., Garhart, E., Leinenweber, K., Prakapenka, V., Greenberg, E., & Shim, S. H. (2020). Oxidation of the interiors of carbide exoplanets. The Planetary Science Journal, 1(2), 39.
40 Hakim, K., Spaargaren, R., Grewal, D. S., Rohrbach, A., Berndt, J., Dominik, C., & Van Westrenen, W. (2019). Mineralogy, structure, and habitability of carbon-enriched rocky exoplanets: a laboratory approach. Astrobiology, 19(7), 867-884.
41 Dangi, B. B., Kim, Y. S., Krasnokutski, S. A., Kaiser, R. I., & Bauschlicher Jr, C. W. (2015). Toward the Formation of Carbonaceous Refractory Matter in High Temperature Hydrocarbon-Rich Atmospheres of Exoplanets Upon Micrometeoroid Impact. The Astrophysical Journal, 805(1), 76.
42 Futó, P. P. POSSIBLE FORMATION SCENARIOS AND MINERALOGICAL TYPES OF CARBON-RICH SOLID.
43 Maynard-Casely, H. E., Cable, M. L., Malaska, M. J., Vu, T. H., Choukroun, M., & Hodyss, R. (2018). Prospects for mineralogy on Titan. American Mineralogist: Journal of Earth and Planetary Materials, 103(3), 343-349.
44 Lopes, R. M., & Williams, D. A. (2015). Volcanism on Io. In The encyclopedia of volcanoes (pp. 747-762). Academic Press.
45 Essack, Z., Seager, S., & Pajusalu, M. (2020). Low-albedo surfaces of lava worlds. The Astrophysical Journal, 898(2), 160.
46 O’Malley-James, J. T., & Kaltenegger, L. (2018). Biofluorescent worlds: Global biological fluorescence as a biosignature. Monthly Notices of the Royal Astronomical Society, 481(2), 2487-2496.
47 Huang, Y. F., & Yu, Y. B. (2017). Searching for strange quark matter objects in exoplanets. The Astrophysical Journal, 848(2), 115.
48 Zapata, J., & Negreiros, R. (2020). Orbital Properties and Gravitational-wave Signatures of Strangelet Crystal Planets. The Astrophysical Journal, 892(1), 67.
49 Kuerban, A., Geng, J. J., Huang, Y. F., Zong, H. S., & Gong, H. (2020). Close-in Exoplanets as Candidates for Strange Quark Matter Objects. The Astrophysical Journal, 890(1), 41.
50 Alford, M. G., Han, S., & Reddy, S. (2012). Strangelet dwarfs. Journal of Physics G: Nuclear and Particle Physics, 39(6), 065201.
51 Lammer, H., Stökl, A., Erkaev, N. V., Dorfi, E. A., Odert, P., Güdel, M., ... & Leitzinger, M. (2014). Origin and loss of nebula-captured hydrogen envelopes from ‘sub’-to ‘super-Earths’ in the habitable zone of Sun-like stars. Monthly Notices of the Royal Astronomical Society, 439(4), 3225-3238.
52 Wang, Z., Zhou, Y., & Liu, Y. (2022). The escape mechanisms of the proto-atmosphere on terrestrial planets:“boil-off” escape, hydrodynamic escape and impact erosion. Acta Geochimica, 41(4), 592-606.
53 Williams, D. M., Kasting, J. F., & Wade, R. A. (1997). Habitable moons around extrasolar giant planets. Nature, 385(6613), 234-236.
54 Zahnle, K. J., Lupu, R., Catling, D. C., & Wogan, N. (2020). Creation and evolution of impact-generated reduced atmospheres of early Earth. The Planetary Science Journal, 1(1), 11.
55 Gunell, H., Maggiolo, R., Nilsson, H., Wieser, G. S., Slapak, R., Lindkvist, J., ... & De Keyser, J. (2018). Why an intrinsic magnetic field does not protect a planet against atmospheric escape. Astronomy & Astrophysics, 614, L3.
56 Swain, M. R., Estrela, R., Roudier, G. M., Sotin, C., Rimmer, P. B., Valio, A., ... & Zellem, R. T. (2021). Detection of an atmosphere on a rocky exoplanet. The Astronomical Journal, 161(5), 213.
57 Wordsworth, R., & Pierrehumbert, R. (2013). Hydrogen-nitrogen greenhouse warming in Earth's early atmosphere. science, 339(6115), 64-67.
58 Visions 2200, "Extrasolar Speculations"; further details unclear but it appears to be stably archived.
59 Sudarsky, D., Burrows, A., & Pinto, P. (2000). Albedo and reflection spectra of extrasolar giant planets. The Astrophysical Journal, 538(2), 885.
60 Gao, P., Marley, M. S., Zahnle, K., Robinson, T. D., & Lewis, N. K. (2017). Sulfur hazes in giant exoplanet atmospheres: impacts on reflected light spectra. The Astronomical Journal, 153(3), 139.
61 Parmentier, V., Fortney, J. J., Showman, A. P., Morley, C., & Marley, M. S. (2016). Transitions in the cloud composition of hot Jupiters. The Astrophysical Journal, 828(1), 22.
62 "Neptunian and Jovian Cloud Types for Orion's Arm", google doc; if this disappears, check out the EWoCS page on Orion's Arm based on it https://orionsarm.com/eg-article/5e724eb65b934
63 Helled, R., Movshovitz, N., & Nettelmann, N. (2022). The nature of gas giant planets. arXiv preprint arXiv:2202.10046.
64 Hu, R., Seager, S., & Yung, Y. L. (2015). Helium atmospheres on warm Neptune-and sub-Neptune-sized exoplanets and applications to GJ 436b. The Astrophysical Journal, 807(1), 8.
65 Bailes, M., Bates, S. D., Bhalerao, V., Bhat, N., Burgay, M., Burke-Spolaor, S., ... & van Straten, W. (2011). Transformation of a star into a planet in a millisecond pulsar binary. Science, 333(6050), 1717-1720.
66 Hu, R., & Seager, S. (2014). Photochemistry in terrestrial exoplanet atmospheres. III. Photochemistry and thermochemistry in thick atmospheres on super Earths and mini Neptunes. The Astrophysical Journal, 784(1), 63.
67 Hu, R., & Thomas, T. B. (2022). A nitrogen-rich atmosphere on ancient Mars consistent with isotopic evolution models. Nature Geoscience, 15(2), 106-111.
68 Mikhail, S., & Sverjensky, D. A. (2014). Nitrogen speciation in upper mantle fluids and the origin of Earth's nitrogen-rich atmosphere. Nature Geoscience, 7(11), 816-819.
69 Catling, D. C., & Zahnle, K. J. (2020). The archean atmosphere. Science advances, 6(9), eaax1420.
70 Wordsworth, R. D. (2016). Atmospheric nitrogen evolution on Earth and Venus. Earth and Planetary Science Letters, 447, 103-111.
71 Laneuville, M., Kameya, M., & Cleaves, H. J. (2018). Earth without life: A systems model of a global abiotic nitrogen cycle. Astrobiology, 18(7), 897-914.
72 Krissansen-Totton, J., Schwieterman, E. W., Charnay, B., Arney, G., Robinson, T. D., Meadows, V., & Catling, D. C. (2016). Is the Pale Blue Dot unique? Optimized photometric bands for identifying Earth-like exoplanets. The Astrophysical Journal, 817(1), 31.
73 Meadows, V. S., Reinhard, C. T., Arney, G. N., Parenteau, M. N., Schwieterman, E. W., Domagal-Goldman, S. D., ... & Grenfell, J. L. (2018). Exoplanet biosignatures: understanding oxygen as a biosignature in the context of its environment. Astrobiology, 18(6), 630-662.
74 Watanabe, Y., & Tajika, E. (2021). Atmospheric oxygenation of the early earth and earth-like planets driven by competition between land and seafloor weathering. Earth, Planets and Space, 73(1), 1-10.
75 Krissansen‐Totton, J., Fortney, J. J., Nimmo, F., & Wogan, N. (2021). Oxygen False Positives on Habitable Zone Planets Around Sun‐Like Stars. AGU Advances, 2(2), e2020AV000294.
76 Wordsworth, R., & Pierrehumbert, R. (2014). Abiotic oxygen-dominated atmospheres on terrestrial habitable zone planets. The Astrophysical Journal Letters, 785(2), L20.
77 kumar Kopparapu, R., Wolf, E. T., Arney, G., Batalha, N. E., Haqq-Misra, J., Grimm, S. L., & Heng, K. (2017). Habitable moist atmospheres on terrestrial planets near the inner edge of the habitable zone around M dwarfs. The Astrophysical Journal, 845(1), 5.
78 do Amaral, L. N., Barnes, R., Segura, A., & Luger, R. (2022). The Contribution of M-dwarf Flares to the Thermal Escape of Potentially Habitable Planet Atmospheres. The Astrophysical Journal, 928(1), 12.
79 Hu, R., Peterson, L., & Wolf, E. T. (2020). O2-and CO-rich atmospheres for potentially habitable environments on TRAPPIST-1 planets. The Astrophysical Journal, 888(2), 122.
80 Narita, N., Enomoto, T., Masaoka, S., & Kusakabe, N. (2015). Titania may produce abiotic oxygen atmospheres on habitable exoplanets. Scientific reports, 5(1), 13977.
81 Belcher, C. M., Yearsley, J. M., Hadden, R. M., McElwain, J. C., & Rein, G. (2010). Baseline intrinsic flammability of Earth’s ecosystems estimated from paleoatmospheric oxygen over the past 350 million years. Proceedings of the National Academy of Sciences, 107(52), 22448-22453.
82 Vidaurri, M. R., Bastelberger, S. T., Wolf, E. T., Domagal-Goldman, S., & Kopparapu, R. K. (2022). The Outer Edge of the Venus Zone around Main-sequence Stars. The Planetary Science Journal, 3(6), 137.
83 He, C., Hörst, S. M., Lewis, N. K., Yu, X., Moses, J. I., McGuiggan, P., ... & Vuitton, V. (2020). Sulfur-driven haze formation in warm CO2-rich exoplanet atmospheres. Nature Astronomy, 4(10), 986-993.
84 Schaefer, L., & Fegley, B. (2011). Atmospheric chemistry of Venus-like exoplanets. The Astrophysical Journal, 729(1), 6.
85 Grenfell, J. L., Leconte, J., Forget, F., Godolt, M., Carrión-González, Ó., Noack, L., ... & Turbet, M. (2020). Possible Atmospheric Diversity of Low Mass Exoplanets–Some Central Aspects. Space Science Reviews, 216, 1-38.
86 Zhan, Z., Huang, J., Seager, S., Petkowski, J. J., & Ranjan, S. (2022). Organic Carbonyls Are Poor Biosignature Gases in Exoplanet Atmospheres but May Generate Significant CO. The Astrophysical Journal, 930(2), 133.
87 Needham, D. H., & Kring, D. A. (2017). Lunar volcanism produced a transient atmosphere around the ancient Moon. Earth and Planetary Science Letters, 478, 175-178.
88 Schwieterman, E. W., Olson, S. L., Pidhorodetska, D., Reinhard, C. T., Ganti, A., Fauchez, T. J., ... & Lyons, T. W. (2022). Evaluating the Plausible Range of N2O Biosignatures on Exo-Earths: An Integrated Biogeochemical, Photochemical, and Spectral Modeling Approach. The Astrophysical Journal, 937(2), 109.
89 Airapetian, V. S., Barnes, R., Cohen, O., Collinson, G. A., Danchi, W. C., Dong, C. F., ... & Yamashiki, Y. (2020). Impact of space weather on climate and habitability of terrestrial-type exoplanets. International Journal of Astrobiology, 19(2), 136-194.
90 Lammer, H., Schiefer, S. C., Juvan, I., Odert, P., Erkaev, N. V., Weber, C., ... & Hanslmeier, A. (2014). Origin and stability of exomoon atmospheres: implications for habitability. Origins of life and Evolution of Biospheres, 44, 239-260.
91 Pierrehumbert, R. T., & Ding, F. (2016). Dynamics of atmospheres with a non-dilute condensible component. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 472(2190), 20160107.
92 Kuchner, M. J. (2003). Volatile-rich Earth-mass planets in the habitable zone. The Astrophysical Journal, 596(1), L105.
93 Kaltenegger, L., Sasselov, D., & Rugheimer, S. (2013). Water-planets in the habitable zone: atmospheric chemistry, observable features, and the case of Kepler-62e and-62f. The Astrophysical Journal Letters, 775(2), L47.
94 Goldblatt, C. (2015). Habitability of waterworlds: runaway greenhouses, atmospheric expansion, and multiple climate states of pure water atmospheres. Astrobiology, 15(5), 362-370.
95 Arnscheidt, C. W., Wordsworth, R. D., & Ding, F. (2019). Atmospheric evolution on low-gravity waterworlds. The Astrophysical Journal, 881(1), 60.
96 Del Genio, A. D., Kiang, N. Y., Way, M. J., Amundsen, D. S., Sohl, L. E., Fujii, Y., ... & Kelley, M. (2019). Albedos, equilibrium temperatures, and surface temperatures of habitable planets. The Astrophysical Journal, 884(1), 75.
97 Pluriel, W., Marcq, E., & Turbet, M. (2019). Modeling the albedo of Earth-like magma ocean planets with H2O-CO2 atmospheres. Icarus, 317, 583-590.
98 Liggins, P., Jordan, S., Rimmer, P. B., & Shorttle, O. (2022). Growth and evolution of secondary volcanic atmospheres: I. Identifying the geological character of hot rocky planets. Journal of Geophysical Research: Planets, 127(7), e2021JE007123.
99 Civiš, S., Knížek, A., Ivanek, O., Kubelík, P., Zukalová, M., Kavan, L., & Ferus, M. (2017). The origin of methane and biomolecules from a CO2 cycle on terrestrial planets. Nature Astronomy, 1(10), 721-726.
100 Haqq-Misra, J. D., Domagal-Goldman, S. D., Kasting, P. J., & Kasting, J. F. (2008). A revised, hazy methane greenhouse for the Archean Earth. Astrobiology, 8(6), 1127-1137.
101 Crow, C. A., McFadden, L. A., Robinson, T., Meadows, V. S., Livengood, T. A., Hewagama, T., ... & Wellnitz, D. (2011). Views from EPOXI: colors in our solar system as an analog for extrasolar planets. The Astrophysical Journal, 729(2), 130.
102 Hu, R., Seager, S., & Bains, W. (2013). Photochemistry in terrestrial exoplanet atmospheres. II. H2S and SO2 photochemistry in anoxic atmospheres. The Astrophysical Journal, 769(1), 6.
103 Loftus, K., Wordsworth, R. D., & Morley, C. V. (2019). Sulfate aerosol hazes and SO2 gas as constraints on rocky exoplanets’ surface liquid water. The Astrophysical Journal, 887(2), 231.
104 Zilinskas, M., Miguel, Y., Mollière, P., & Tsai, S. M. (2020). Atmospheric compositions and observability of nitrogen-dominated ultra-short-period super-Earths. Monthly Notices of the Royal Astronomical Society, 494(1), 1490-1506.
105 Fegley, B., Jacobson, N. S., Williams, K. B., Plane, J. M. C., Schaefer, L., & Lodders, K. (2016). Solubility of rock in steam atmospheres of planets. The Astrophysical Journal, 824(2), 103.
106 Schaefer, L., & Fegley, B. (2009). Chemistry of silicate atmospheres of evaporating super-Earths. The Astrophysical Journal, 703(2), L113.
107 Lammer, H., Scherf, M., Ito, Y., Mura, A., Vorburger, A., Guenther, E., ... & Odert, P. (2022). The exosphere as a boundary: Origin and evolution of airless bodies in the inner solar system and beyond including planets with silicate atmospheres. Space science reviews, 218(3), 15.
108 Ranjan, S., Seager, S., Zhan, Z., Koll, D. D., Bains, W., Petkowski, J. J., ... & Lin, Z. (2022). Photochemical Runaway in Exoplanet Atmospheres: Implications for Biosignatures. The Astrophysical Journal, 930(2), 131.
109 Rimmer, P. B., & Rugheimer, S. (2019). Hydrogen cyanide in nitrogen-rich atmospheres of rocky exoplanets. Icarus, 329, 124-131.
110 Luque, R., & Pallé, E. (2022). Density, not radius, separates rocky and water-rich small planets orbiting M dwarf stars. Science, 377(6611), 1211-1214.
111 Madhusudhan, N., Piette, A. A., & Constantinou, S. (2021). Habitability and biosignatures of Hycean worlds. The Astrophysical Journal, 918(1), 1.
112 Cowan, N. B., & Abbot, D. S. (2014). Water cycling between ocean and mantle: super-Earths need not be waterworlds. The Astrophysical Journal, 781(1), 27.
113 Schaefer, L., & Sasselov, D. (2015). The persistence of oceans on Earth-like planets: Insights from the deep-water cycle. The Astrophysical Journal, 801(1), 40.
114 Noack, L., Höning, D., Rivoldini, A., Heistracher, C., Zimov, N., Journaux, B., ... & Bredehöft, J. H. (2016). Water-rich planets: How habitable is a water layer deeper than on Earth?. Icarus, 277, 215-236.
115 Nixon, M. C., & Madhusudhan, N. (2021). How deep is the ocean? Exploring the phase structure of water-rich sub-Neptunes. Monthly Notices of the Royal Astronomical Society, 505(3), 3414-3432.
116 Renno, N. O., Fischer, E., Martínez, G., & Hanley, J. (2021). Complex Brines and Their Implications for Habitability. Life, 11(8), 847.
117 Halevy, I., Alesker, M., Schuster, E. M., Popovitz-Biro, R., & Feldman, Y. (2017). A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nature Geoscience, 10(2), 135-139.
118 Gallardo, V. A., & Espinoza, C. (2008, August). The evolution of ocean color. In Instruments, Methods, and Missions for Astrobiology XI (Vol. 7097, pp. 128-134). SPIE.
119 Gueneli, N., McKenna, A. M., Ohkouchi, N., Boreham, C. J., Beghin, J., Javaux, E. J., & Brocks, J. J. (2018). 1.1-billion-year-old porphyrins establish a marine ecosystem dominated by bacterial primary producers. Proceedings of the National Academy of Sciences, 115(30), E6978-E6986.
120 Cordier, D., Mousis, O., Lunine, J. I., Lebonnois, S., Rannou, P., Lavvas, P., ... & Ferreira, A. G. M. (2012). Titan's lakes chemical composition: sources of uncertainties and variability. Planetary and Space Science, 61(1), 99-107.
121 Hayes, A. G., Lorenz, R. D., & Lunine, J. I. (2018). A post-Cassini view of Titan’s methane-based hydrologic cycle. Nature Geoscience, 11(5), 306-313.
122 Faulk, S. P., Lora, J. M., Mitchell, J. L., & Milly, P. C. D. (2020). Titan’s climate patterns and surface methane distribution due to the coupling of land hydrology and atmosphere. Nature Astronomy, 4(4), 390-398.
123 Cordier, D., & Carrasco, N. (2019). The floatability of aerosols and wave damping on Titan’s seas. Nature Geoscience, 12(5), 315-320.
124 GLIESE, S. S. M. O., & d Steven, M. AN INVESTIGATION OF EXTENSIVE TIDALLY HEATED SUPER-EARTHS (SUPER-IOS) USING A. Icarus, 226, 1743-1761.
125 Woitke, P., Herbort, O., Helling, C., Stüeken, E., Dominik, M., Barth, P., & Samra, D. (2021). Coexistence of CH4, CO2, and H2O in exoplanet atmospheres. Astronomy & Astrophysics, 646, A43.
126 Hogenboom, D. L., Kargel, J. S., Holden, T. C., & Ganasan, J. (1994, March). The ammonia-water phase diagram and phase volumes to 4 kbars. In Lunar and Planetary Science Conference (Vol. 25, p. 555).
127 Graham, R. J., Lichtenberg, T., & Pierrehumbert, R. T. (2022). CO2 ocean bistability on terrestrial exoplanets. Journal of Geophysical Research: Planets, 127(10), e2022JE007456.
128 Tang, Y., Chen, Q., & Huang, Y. (2006). Early Mars may have had a methanol ocean. Icarus, 180(1), 88-92.
129 Schulze-Makuch, D., Irwin, L. N., Schulze-Makuch, D., & Irwin, L. N. (2018). Life and the Need for a Solvent. Life in the universe: Expectations and constraints, 123-147.
130 Dougherty, A. J., Bartholet, Z. T., Chumsky, R. J., Delano, K. C., Huang, X., & Morris, D. K. (2018). The Liquidus Temperature for Methanol‐Water Mixtures at High Pressure and Low Temperature, With Application to Titan. Journal of Geophysical Research: Planets, 123(12), 3080-3087.
131 Saladino, R., Crestini, C., Pino, S., Costanzo, G., & Di Mauro, E. (2012). Formamide and the origin of life. Physics of life reviews, 9(1), 84-104.
132 Miyazaki, Y., & Korenaga, J. (2022). Inefficient water degassing inhibits ocean formation on rocky planets: An insight from self-consistent mantle degassing models. Astrobiology, 22(6), 713-734.
133 Lupu, R. E., Zahnle, K., Marley, M. S., Schaefer, L., Fegley, B., Morley, C., ... & Fortney, J. J. (2014). The atmospheres of earthlike planets after giant impact events. The Astrophysical Journal, 784(1), 27.
134 Nikolaou, A., Katyal, N., Tosi, N., Godolt, M., Grenfell, J. L., & Rauer, H. (2019). What factors affect the duration and outgassing of the terrestrial magma ocean?. The Astrophysical Journal, 875(1), 11.
135 Hamano, K., Kawahara, H., Abe, Y., Onishi, M., & Hashimoto, G. L. (2015). Lifetime and spectral evolution of a magma ocean with a steam atmosphere: its detectability by future direct imaging. The Astrophysical Journal, 806(2), 216.
136 Rappaport, S., Levine, A., Chiang, E., El Mellah, I., Jenkins, J., Kalomeni, B., ... & Tran, K. (2012). Possible disintegrating short-period super-Mercury orbiting KIC 12557548. The Astrophysical Journal, 752(1), 1.
137 Léger, A., Grasset, O., Fegley, B., Codron, F., Albarede, A. F., Barge, P., ... & Sotin, C. (2011). The extreme physical properties of the CoRoT-7b super-Earth. Icarus, 213(1), 1-11.
138 Boukaré, C. É., Cowan, N. B., & Badro, J. (2022). Deep two-phase, hemispherical magma oceans on lava planets. The Astrophysical Journal, 936(2), 148.
139 Kite, E. S., Fegley Jr, B., Schaefer, L., & Gaidos, E. (2016). Atmosphere-interior exchange on hot, rocky exoplanets. The Astrophysical Journal, 828(2), 80.
140 Xu, Y., Xiao, X., Sun, S., & Ouyang, Z. (1996, March). IR spectroscopic evidence of metal carbonyl clusters in the Jiange H5 chondrite. In Lunar and Planetary Science Conference (Vol. 27).
141 Schulze-Makuch, D. (2013). Extremophiles on alien worlds: what types of organismic adaptations are feasible on other planetary bodies. Habitability of Other Planets and Satellites, 253-265.
142 Bains, W. (2004). Many chemistries could be used to build living systems. Astrobiology, 4(2), 137-167.
143 Flower, P. J. (1996). Transformations from theoretical Hertzsprung-Russell diagrams to color-magnitude diagrams: effective temperatures, BV colors, and bolometric corrections. Astrophysical Journal v. 469, p. 355, 469, 355.
144 "Locating Geosynchronous Satellites", Australian Space Academy
Previous versions:























Hello. I'm reasonably sure the formula for maximum elevation above the horizon of an equatorial satellite is incorrect, as it gives incorrect results when you plug values for various planet-satellite systems. Could you look into it please?
ReplyDeleteYeah, can't remember where I got that from, but I've replaced it with one from here https://www.spaceacademy.net.au/watch/track/locgsat.htm
Delete"fires become near impossible below 16% atmospheric oxygen, allowing levels to rise unhindered, and near inevitable above 22%"
ReplyDeleteIs 16 and 22% oxygen content for any atmosphere, or is it 0.16 and 0.22 atm of partial pressure of oxygen?
Moreso the latter. The level of background gasses probably does have some impact, but for, say, a 10 atm atmosphere, I'd expect a similar level of flammability to be encountered closer to 0.2 atm oxygen than 2 atm.
DeleteWhat about the oxygen/carbon dioxide ratio? I've heard the ratio is more important than raw concentration when it comes to oxygen breathing animals, so I would assume perhaps the same for fire?
DeleteInteresting stuff! I've read about the shift from rocky to sub-Neptune at around 1.2 times Earth radius and 2 times Earth mass. I'm having a hard time parsing the chart on that - is it a very sudden transition beyond that threshold, or a gradual one. Would we, for example, expect a fair number of planets with something like 1.5 times Earth radius to be rocky while almost none at 2 times Earth radius would be, or would the 1.5 times Earth radius planets be almost all sub-Neptunes?
ReplyDeleteThe chart at the top is showing an attempt to fit lines to the trends; the circles in it are the actual data from exoplanets. As you can see, there's a fair bit of scatter, so there are probably some rocky planets above that transition. I'd be a bit surprised if there wasn't at least a couple rocky planets over 2 radii, but there wouldn't be many. 1.5 to 2 Earth radii is quite the jump, mind; at Earth's composition, they correspond roughly to 4.5 and 16 Earth masses.
DeleteIt is a very gradual transition, to rocky or ocean planet with an Earth-like atmosphere, to a gradually thicker methane-dominated atmosphere, to hydrogen/helium-dominated, to eventually the atmosphere becoming so thick that the lower layers become supercritical fluid and the surface/atmosphere distinction is erased. Atmospheres are held onto much more strongly in cold planets, so an Earth-size planet in Pluto's orbit would very likely already be a sub-Neptune, while you could have a planet twice the radius of Earth still be rocky in Mercury's orbit.
DeleteVery intertesting article! Shor question - I consider that I put in to my world hot Neptune or hot Jupiter. Would it be visible from habbitable planet during a sunrise/sunset just with naked eye? This could have some interesting worldbuilding implications. Some more advanced civilazations could make some special materials which block most of the sunlight and observe hot Jupiter that way, but it would be more interesting if ancient civilazations can see it too.
ReplyDeleteHow come Teacup Ac's atmosphere is dominated by N2 instead of CO2?
ReplyDeleteI'm specifically going for a type of marginally habitable close-orbiting desert planet that I discuss in the next post which requires very low levels of atmospheric water and CO2; we can suppose this is maintained by low rates of volcanism.
DeleteAre natural planets made mostly of radioactive material possible?
ReplyDeleteFor any substantially radioactive material (i.e. setting aside technicalities like ever-so-slightly radioactive bismuth) the waste heat produced inside the planet from decay would probably be enough to blow the planet apart, or at the very least do something unpleasant to its surface. That issue aside, it'd be unlikely to form at all because radioactive materials aren't chemically distinctive enough to be sorted out on that scale by any naturally process, and remain fairly rare because they keep decaying away. Various potential scenarios could perhaps cause concentrations of highly radioactive material to be present in pockets on a planet's surface, but it's hard to say what the limits there are in terms of how widespread these pockets would be, how radioactive, and for how long of a planet's lifetime. Continuous bombardment by an external source of ionizing radiation is rather easier to achieve.
DeleteLike being formed from supernova debris in a neutron star or black hole system or getting radiated by pulsar, x-ray or gamma ray blast?
Delete"I wouldn't be especially surprised to encounter a world half Earth's mass with a greater surface pressure or one twice Earth's mass with less."
ReplyDeleteCongratulations, you just described Aegis (it has a surface gravity of 0.8 g, 0.51 earth masses, and 1.2 atm of pressure)
Hello, sorry to bother. I’ve been having fun worldbuilding a planet with a near-100% oxygen atmosphere, but I have a few small questions related to this blog. The planet (which orbits an F-star) has 2.5 atms of sea-level pressure. This is far too toxic to breathe, but the continents all have plateaus that are ~7 kilometers above sea level, where the pressure has dropped to (a still-barely habitable) 0.45 atms. (I am unconcerned with the plausibility of the abundance of oxygen or the implausibly high continents, as in-universe, the planet is a young and constructed, with a dead interior). However, I have two questions related to this blog:
ReplyDeleteFirstly, if I were to try to model the climate with ExoPlaSim, do you think I would get more accurate results if I modeled the planet as it really is (a sea level pressure of 2.5 atms with kilometers-tall continents), or if I modeled the planet pretending that the sea level pressure is 0.45 atms with normal-elevation continents? Is ExoPlaSim capable of realistic outputs with elevations like that, and/or such an oxygen-rich atmosphere?
Secondly, I noticed the formula for pressure-at-given-altitude that you provided in IVb. However, I noticed that your formula includes Euler’s number, and my interpretation of the “Scale height” Wikipedia page is that atmospheric pressure only decreases by a factor of Euler’s number until the Scale Height is reached; and that pressure doesn’t follow that factor anymore above the Scale Height. But I might be misunderstanding the Wikipedia page. Do you know if your pressure-at-a-given altitude formula from IVb is accurate above the Scale Height? If it is, until what alitutude is it accurate until?
Once again, sorry for the bother and thank you in advance.
I've run Exoplasim models with plateaus of 8-10 km, and I know other people have run models on maps where they lowered Earth's sea levels by several km, so the model should generally be capable of large elevation ranges like that. The pressure you input would correspond to wherever zero elevation is, and the model will figure out pressure variation at other elevations; there's no particular reason you can't have negative elevations, so you could call the tops of the plateaus zero elevation if you liked and just have deep pits elsewhere, but I haven't really tested that sort of approach so I suppose my instinct would be to keep zero elevation at sea level.
DeleteThe issue you might have is that exoplasim sometimes has issues with sharp elevation changes; so if you get consistent crashes you might try lowering the timestep first to see if that helps, and then if it still crashes you may have to smooth out some of your slopes a bit.
It shouldn't have any issue with an oxygen-dominated atmosphere, so far as the model is concerned the only difference between nitrogen and oxygen is that the latter has a slightly higher molar mass.
I'm not sure where you're getting that about scale height, nothing special happens at the scale height altitude itself, it's just a mathematically convenient measure of the overall pressure-altitude curve. In an idealized case the pressure should follow the same exponential curve to essentially infinite altitude, but in reality there will be some deviation due to changing temperature with altitude and decreasing gravity with height, and at sufficiently low pressures (somewhere around the karman line) different gas species start behaving more independently and interactions with solar wind become more significant. So that equation isn't a perfect approximation but should tend to work well enough throughout the lower atmosphere.
Some late breaking news: It’s recently discovered that Mercury is leaving a trail of sodium vapor
ReplyDeletehttps://youtu.be/7d87NF8cKm8?si=qoh9I5HkKYxKT3Hg
Is it possible to have naturally occurring metallic (gallium, mercury) or iodine large bodies of liquid? Since they are rare heavy elements, they require a second-generation planet to form?
ReplyDeleteHave you responded to my question yet?
DeleteI wonder why you still haven't addressed the specific atmospheric composition of Teacup Ae. Because it is likely a crucial worldbuilding detail.
ReplyDelete2-bar nitrogen-dominated is the most important detail, I wanted to wait to set an O2 level until I talked a little more about the biological implications of O2 in a later post but it'll probably be about the same as Earth, CO2 level may depend on some final refinements to the climate but seems to be coming out as somewhere around 0.02 bar, any other minor gasses aren't terribly important to pin down.
DeleteAround when you talk about the implications of various O2 levels? And will it be in another "Apple Pie From Scratch"?
DeleteAround when you address the implications of various O2 levels? And will it be in another "Apple Pie From Scratch"?
ReplyDeletePlanetsynth no longer works anymore.
ReplyDeleteI'm a little confused as to the lack of discussion on the specifics of planetary composition. I've looked into this myself and it seems like there are both certain things which constrain relative abundances as well as degrees of freedom. But even if terrestrial planets are constrained to strictly earth-like ratios it's confusing that this isn't clarified or explained. At the beginning of the Rock section it is stated "For a system with a composition like ours, rocky material consists mostly of silica (SiO2) mixed with magnesium, calcium, and aluminum" but then very little discussion is had about what other possible types of compositions there are, or what processes restrict the possible compositions. This is a difficult subject to look into so I'm still trying to figure it out myself, it seems like some of the major restrictions are overall universal abundance and tendency to being rock forming, whereas degrees of freedom come in the composition of the original planetary nebula (i can't tell how much this can vary, different papers say different things) and relative proportions to some degree.
ReplyDeleteMainly it's because I've generally tried not to speculate too far beyond the available literature, and that is quite limited. Between the bulk composition of a protoplanetary disk and the details of the surface environment of a mature planet are numerous complex processes that are all only partially understood. Most studies on the subject basically take the example of what we can see in the solar system and then try to imagine variations on that based to a greater or lesser extent on what limited data we have on exoplanets. A few have been a little more speculative, and I've tried to seek those out, but I'm ultimately limited by what specific speculation some researcher has decided to publish.
DeleteThere are some reasons to expect variation to be somewhat limited though. Relative abundance of many elements probably doesn't vary much throughout the universe, because they're ultimately controlled by quite reliable stellar nucleosynthesis processes, and then certain groups of elements have a chemical affinity to each other and similar physical properties, so are unlikely to be separated at any stage of planet formation, and so their relative abundance will remain much the same, which thus reliably informs the resulting planetary chemistry. So for example the processes that form manganese also form much more iron, and manganese and iron will tend to stick together. A planet may vary in how much overall manganese+iron it gets, but there's no way you'd ever really expect to get a lot of manganese but not lots and lots of iron. Thus on a planetary scale manganese will pretty much always be essentially an impurity in a much larger mass of iron, and so you can largely ignore its effect on the overall planetary chemistry. It's like if any time I used green paint, I then went over it with ten times more blue paint; I'll never make a painting that features green as a major color.
Thus the choice to divide up planetary composition into these major categories of volatiles, rocks, and metals. There's a bit more variation in behavior within volatiles, and then sulfur-like elements can sometimes make their own category, but you never really expect to see any rocky material that isn't dominated by some combination of silicon, magnesium, oxygen, and carbon, and you never expect to see much of the metals without it being mostly iron. There are a number of special cases I made notes of and we could find more in the future, but I don't think we're ever going to see a planet with a copper core or a bromide crust.
That all makes sense, although I'm curious as to the specifics. Why exactly are silicon and magnesium the primary rock-forming culprits? What's special about them chemically compared to other common non-volatile elements? I think this is more of a basic chemistry question than a planetary geology question but I'm having a hard time finding a straight answer. Most papers seem to just assume this so I think it must be really basic, but I can't find explanations of it.
DeleteAnd what about Calcium and Aluminum? They're significant as well but is that for similar deterministic reasons or might that vary?
Hmm, you may have a point that some sort of little intro section about this may be useful, but let me try a short version: Basically you start with the initial abundance of chemical elements throughout the early solar system ( https://en.wikipedia.org/wiki/Abundance_of_the_chemical_elements#/media/File:Elements_abundance-bars.svg ; note the logarithmic scale), and then for an Earthlike planet a number of processes remove some of the common ones:
Delete- hydrogen mostly forms hydrogen gas and helium bonds with nothing, so these are mostly swept out of the inner system by solar wind and so on, with a little hydrogen maybe remaining to bond with other volatiles.
- oxygen, carbon, and nitrogen most readily bond with each other or hydrogen, forming materials that will mostly be gasses in the inner system and so are again swept out until they're far enough from the sun to form ice and clump together into comets etc. Oxygen is the most common, so once the other volatiles are gone some is left over and can bond with heavier elements to form rocky oxides.
- neon and argon are noble gasses and so again swept away
- iron, nickel, and sulfur mostly sink into the core of any forming planet, along with various other less common metals
That leaves silicon and magnesium as the most common remaining elements to bond with the leftover oxygen and form the exterior of a small inner system planet, with sodium, aluminum, and calcium as distant runner-ups.
I think an intro section regarding this would be helpful yeah
DeleteI'm trying to find resources on ways that trace gases or aerosols in an atmosphere may affect its color. Like for instance, could a small amount of chlorine gas tint the sky greenish? and how could I calculate how much? I'd expect there to be more alien sky color resources but it's surprisingly sparse. Do you have any ideas or resources about this?
ReplyDelete